Table of Contents (click to expand)
Salts melt snow by lowering its freezing point. Sugar can be used for the same purpose, but there are some caveats to that alternative strategy.
Going sledding, making snowmen, and having snowball fights are some ideal ways to enjoy winter weather. However, you might not feel the same way when that snow covers your driveway, roads, and roofs. It is frustrating and time-consuming to clear out all that snow!
Salts can help us tackle these problems.
Rock salt, also known as Sodium chloride or Brine, and other anhydrous salts like magnesium chloride and calcium chloride, are used in bulk quantities by the government to melt road snow during winter. The salts used for melting snow on pavement are commonly known as ‘Deicers’ or ‘Road Salts’. They cannot be consumed like edible salts.
Recommended Video for you:
How Does Salt Melt Snow?
Water freezes at 00C, and at this temperature, ice and water, i.e., the solid and liquid states, are in equilibrium with each other. When we add salt (any salt) to water, it dissolves in it and causes a phenomenon called ‘Depression in Freezing point’.
This is a colligative property shown by solvents like water. Hence, the water that was supposed to freeze at 00C will now freeze at a lower temperature (say -100C), depending on the salt concentration. So, if the atmospheric temperature is 00C, snow will melt instead of remaining in its solid form.
The snow during winter storms is not 100% solid. It is soft to the touch, which signifies that the water has not completely frozen. The deicers get dissolved in these small water cavities and melt the snow. A small concentration of salt is enough to melt a large chunk of snow. Road salts form a major commercial product in countries with annual snowy winters, like the USA, Canada, United Kingdom, etc. The United States annually utilizes NaCl and other Chloride-based road salts worth roughly 24 million dollars.
Can Sugar Melt Snow?
At the freezing point of water, molecules of water lose their kinetic energy and solidify, forming a crystalline structure. As more and more molecules are attracted, they become compact and develop a strong attraction due to Hydrogen bonding. As seen in the figure, there is a relative decrease in the intermolecular distance when water goes from liquid to ice.
This intermolecular distance is disrupted when we add any soluble solutes, like salts. Molecules that dissolve in water overcome the attraction between water molecules and get squeezed between them. As a result, the attraction between like molecules decreases, and it takes even lower temperatures to solidify.
Sugar, i.e., sucrose, readily dissolves in water, so it can also cause a depression in the freezing point of water. However, sucrose doesn’t dissociate into its constituent ions, but instead remains as an uncharged disaccharide. On the other hand, salts ionize into their constituent ions, e.g., MgCl2 –> Mg2+ + 2Cl–, and easily displace themselves between water molecules.
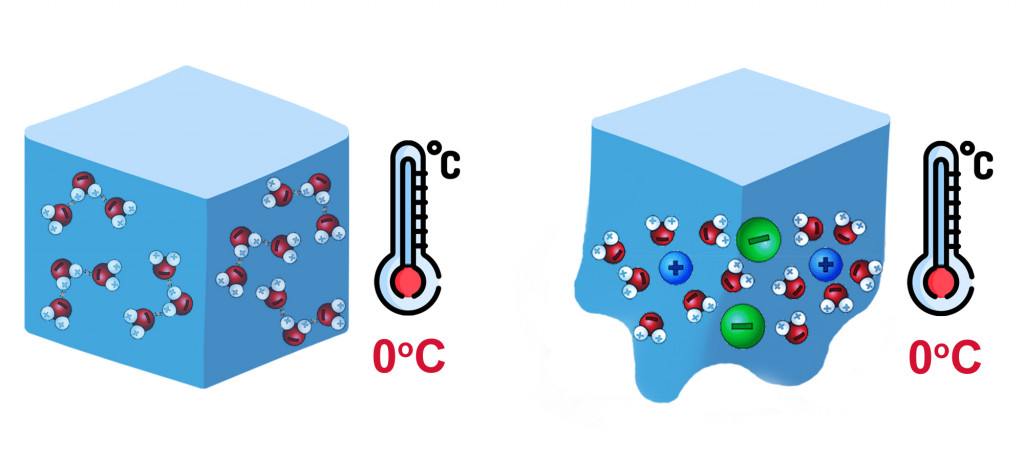
Depression in the freezing point depends on the number of solutes present in the solvent: the more solute particles, the greater the lowering of the freezing point.
Therefore, although sugar can melt snow, it cannot do it as effectively as salts.
Why Are We Looking For Alternatives?
After the snow melts, it is cleared either by the steady stream of vehicles or the flow of water into the nearest drainage system. However, the problem is that the salt on the road can negatively impact the environment.
Most road salts are chloride-based salts. Chloride is a nemesis for vehicles, as it initiates corrosion-induced degradation. Chloride absorbs moisture, thus speeding up the rate of corrosion.

The salts that remain in the water enter nearby water bodies and can disrupt aquatic ecosystems. Water bodies near cities are usually freshwater resources like rivers, lakes, and ponds; if salts enter these reservoirs, they reduce dissolved oxygen (DO) and endanger aquatic life. Increased salinity results in a toxic algal bloom by killing algae-eating zooplankton.
One might think that salts are everywhere in the environment, especially chloride salts, which are essential for living cells. Yes, this is true, but to give you some perspective, these salts are used by the millions of tons each year. Hence, the amount of salt entering the environment untreated is beyond permissible levels. Therefore, we need to look for alternative salts or snow-melting systems that cause less harm to the environment.
What Are Some Other Alternatives?
Commonly used chloride alternatives are:
- Beet sugar
- Glycols
- Molasses
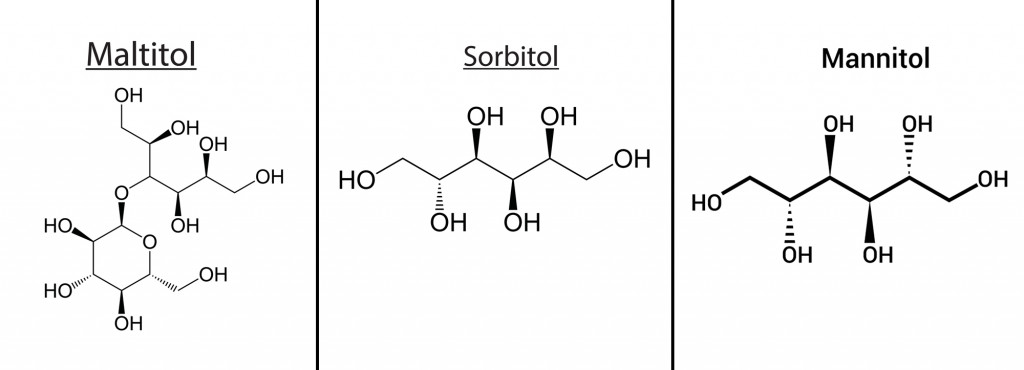
- Corn-derived polyols like mannitol, sorbitol, maltitol
- Hydronic, electric and infrared lamps.
Brine salt is found naturally, while other chloride salts can easily be extracted from minerals. Hence, to minimize the effects of these salts, additives are added instead of bulk-producing new salts.
These additives are bio-based, so they degrade easily in the environment. Beet sugar, glycols, and molasses have been proven to show good deicing properties. Agro-based additives, especially corn-derived polyols like mannitol, sorbitol, and maltitol, have proven to be suitable additives to brine salt, which bring about a good depression in the freezing point of water.
Apart from chemicals, machines can also melt snow. Spraying steam and hot water on accumulated snow proves to be effective and eliminates the manual task of removing snow. Hydronic, electric and infrared lamps are found to do the same for pavement surfaces, sidewalks, and bridge decks.
Finding alternatives for chloride salts has been challenging researchers, mainly for 2 reasons: cost and energy. The additives mentioned above are costly to produce in such bulk quantities and machines are energy-intensive tools. Thus, finding a perfect alternative that ticks all the boxes is an ongoing challenge.
A Final Word
Snow is melted by manipulating its freezing point depression, often through the use of soluble solutes like salts and sugars. The concentration and atmospheric temperature also play an essential role in the effectiveness of these deicers. Commercial NaCl deicer can’t melt ice below -100C. Hence, various salts and additives are added to melt snow in extreme temperatures, while having a less harmful environmental impact.
References (click to expand)
- Ullah Sajid, H., Naik, D. L., & Kiran, R. (2021, February). Improving the ice-melting capacity of traditional deicers. Construction and Building Materials. Elsevier BV.
- Lockwood, D. (2019, March 1). Lakes and Rivers Are Getting Saltier. ACS Central Science. American Chemical Society (ACS).
- Mensah, K., & Choi, J. M. (2015, December). Review of technologies for snow melting systems. Journal of Mechanical Science and Technology. Springer Science and Business Media LLC.
- Bio-Based Renewable Additives for Sustainable Roadway .... transportation.gov












