Lithium ion batteries are recycled using chemical, heat or direct physical methods to separate critical components and reuse them.
Technological advancement and sustainability are the cornerstones of our modern age. With more people making ecologically conscious choices, the world order is witnessing disruption in areas that were previously deemed unshakeable.
One such area is electric mobility. A lot has been discussed about electric vehicles, whether they are truly environmentally friendly and whether they will ever be able to replace fossil fuel-driven vehicles. However, very little is clearly known about battery recycling. Batteries, an essential component of all electric vehicles, are subject to fast-paced development too. Like every other thing, batteries have a life cycle, so what happens to them when they reach the end of their usefulness? Let’s find out!
Recommended Video for you:
Life Cycle Of A Battery
To begin with, it’s important to note that the batteries we’re talking about here are lithium ion (Li-ion) batteries. They find widespread use in almost all appliances, ranging across wireless devices, or in our case, electric cars. They have high energy density and are rechargeable.
Over time, their ability to hold a charge, as well as the ability to recharge, will dwindle, after which they are no longer useful and must be replaced. Battery packs comprise many lithium ion cells connected to each other and hooked up to a battery management system. This system regulates the rate of charging and discharging of individual cells in the battery pack, thereby maximizing their life.
How Are Used Up Batteries Recycled?
The first step to recycling lithium-ion batteries is breaking them down into their constituent elements. A look at a battery’s anatomy will help us understand what these elements are.
Anatomy Of A Lithium-ion Battery
A lithium-ion battery is comprised of an anode, a cathode, a separator and an electrolyte, all of which are contained in a casing. Needless to say, a lithium-ion cell is made of many compounds. The anodes are typically made of graphite, whereas the cathode comprises various lithium oxides and phosphides. These also contain transition metals, such as iron, nickel, manganese and cobalt.
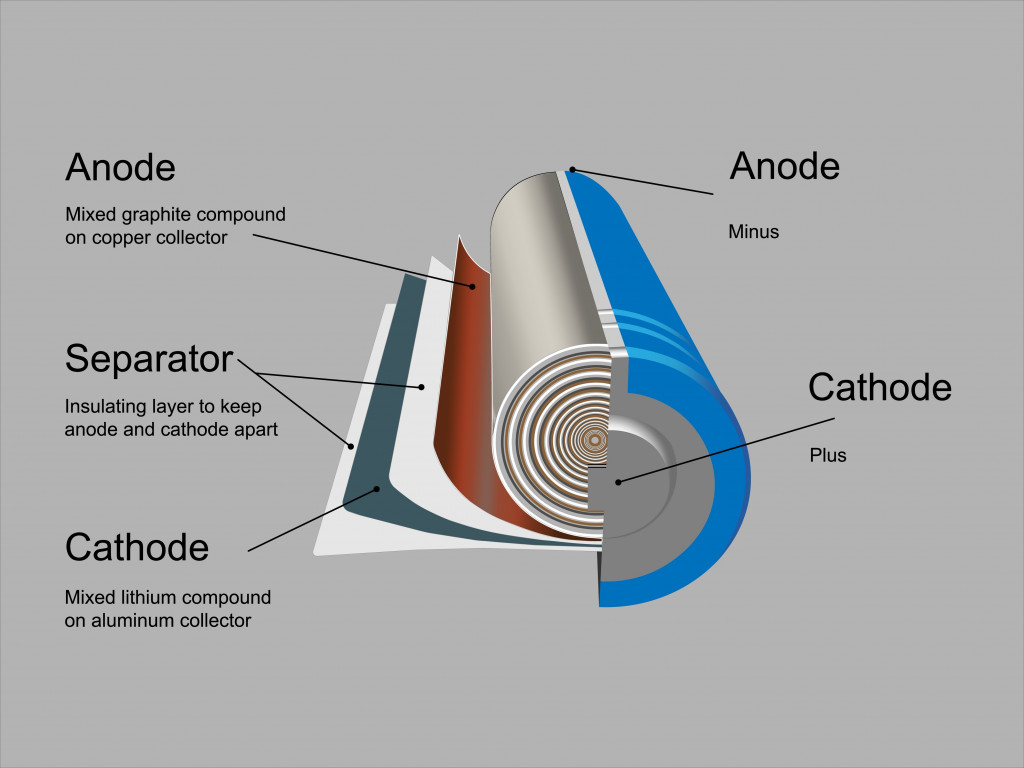
The cathode is the most valuable part of a lithium-ion battery, as most cathodes are comprised of cobalt. Thus, efforts for recycling are hinged around recovering the cathode material and subjecting it to various recycling processes. Over time, however, efforts have been directed towards recovering as much of all the material as economically possible.
Various Methods Of Battery Recycling
Currently, battery recycling is done by three processes. These include hydro-metallurgy, pyro-metallurgy and direct physical recycling.
1. Hydro-metallurgical Recycling
This method of recycling relies heavily on leaching, or treatment with concentrated acids. Batteries are pre-treated to separate plastics and other materials used in the battery. The spent cathode component is treated with various acids to form a metal-ion solution, from which metals can be easily extracted. Commonly used acids include hydrochloric and sulphuric acid. Any residue resulting from the reaction is treated with acid again; this method can extract up to 99% of cobalt and lithium, and 98% of copper.
In newer techniques, weaker acids like oxalic and phosphoric acids are used, yielding elements like manganese and nickel. This method is versatile and can be used for both cobalt and NCM (nickel – copper – manganese) type lithium-ion batteries.
2. Pyro-metallurgical Recycling
The word “pyro” relates to high heat, and pyro-metallurgical recycling is no different. Disassembled batteries are fed into furnaces where they undergo heating at successively increasing temperatures. Lower temperatures bring about the evaporation of the electrolytes (preheating stage) and plastics (pyrolysis stage) present in the battery.

The formation of metal alloys takes place at the highest temperatures in the furnace (smelting stage). Important metals like cobalt, copper and nickel are recovered at this stage, whereas lithium is lost. This method was devised primarily around batteries that are heavily dependent on the use of cobalt. To this day, this remains the most reliable technique for retrieving cobalt from recycled batteries. However, this method is losing relevance, as it does not retrieve lithium, and is practically useless for cobalt-free batteries.
3. Direct Physical Recycling
In physical recycling, batteries are dismantled and treated with CO2 in its supercritical state (no distinct state between liquid and gas). This causes the separation of the electrolyte. While the electrolyte is recovered, the spent cells are further broken down to physically separate metals like copper and aluminum.

The remaining material forms the bulk of the cathode and is sintered at high temperatures to increase their energy density and electrochemical performance. Physical recycling is generally meant for iron- and manganese-based lithium-ion batteries, which are generally free of cobalt.
How Effective Are Recycled Batteries?
As lithium-ion batteries are a new chapter in almost everyone’s life, very little is known about them. Consequently, even less is known about recycling and the effectiveness of the resultant products. Thus, the prevalent notion is that recycled batteries have inferior characteristics to those made of virgin material.
However, this is not true. In various tests, the recycled cathode material was found to be more porous, therefore easing the movement of lithium ions through the electrolyte. They were also found to have similar energy densities, and some even exhibited a longer life cycle. Thus, recycled batteries are on par with their newly mined counterparts.
Advantages And Hurdles To Battery Recycling
While the advantages of battery recycling are based around sustainability, each of the three processes pertaining to battery recycling have their own advantages. At the same time, there are several disadvantages to each process, preventing their large-scale implementation.
While hydro-metallurgical recycling has a high recovery rate and product purity, it is a long process that results in high quantities of waste water generation. Pyro-metallurgical recycling, on the other hand, is a much simpler and shorter operation. On the downside, the process consumes high levels of energy, and does not recover lithium and manganese.
Direct physical recycling is by far the most environmentally friendly process, with a shorter processing time that is not energy intensive. However, the high cost of recovery and incomplete recovery are the biggest downsides there.
End Note – Why Should We Be Recycling Batteries?
An increase in portable devices and battery packs means a major rise in the mining of minerals required to make them. With little to no recycling protocols in place, toxic e-waste resulting from batteries will end up in landfills, leaching chemicals and even causing slow-burning fires.

If we were to solely depend on mining for raw materials, chances are that we would run out, just as we are doing with fossil fuels. Battery recycling technology promises the recovery of almost all components of a battery, which makes it not only sustainable, but lucrative as an industry!
References (click to expand)
- Gaines, L. (2018, September). Lithium-ion battery recycling processes: Research towards a sustainable course. Sustainable Materials and Technologies. Elsevier BV.
- Zhou, L.-F., Yang, D., Du, T., Gong, H., & Luo, W.-B. (2020, December 3). The Current Process for the Recycling of Spent Lithium Ion Batteries. Frontiers in Chemistry. Frontiers Media SA.
- Getting the Lead Out: Why Battery Recycling Is a Global .... Yale Environment 360