Table of Contents (click to expand)
Proteins are measured using the Kjeldahl method and fats are measured by solvent extraction, while carbohydrates and Calories are measured by their analytical values.
The black-and-white nutritional chart provided on food labels is every gym-goer’s best friend. These charts can help you decide whether to pass on the Snickers bar or go for another. The nutritional chart also provides information on the exact number of Calories, proteins, fats and the other nutrients present inside the packaged food. If you’re a fitness freak or just health conscious, you know that everyone talks in terms of total Calories and carbs in different foods.
However, have you ever wondered how such specific numbers are reached? How do manufacturers know exactly how much protein is in a chicken breast? Or how much fat is in one of our beloved doughnuts? Let’s take a closer look at how the three important macronutrients—proteins, fats, and carbohydrates—are measured, in addition to learning how the total Calorie counts are measured!

Recommended Video for you:
Proteins
Proteins are the building blocks of life. They are also among the first things people check for (after fats/sugar) before consuming anything. If you’re a gym-bro, proteins might be the only nutrient you care about!
Proteins, in purely scientific terms, are complex polymer chains made of amino acids and bonded together by peptide bonds. If that’s a bit too much science, just remember that proteins contain nitrogen, while none of the other macronutrients (carbs & fats) do.
The presence of nitrogen in proteins helps us figure out how much protein is available in our foods. The nitrogen content is determined and this amount is then multiplied by a factor to get the protein content. On average, we find the nitrogen content in proteins to be 16%.
So total protein = Nitrogen in Food x 6.25 (1/0.16 = 6.25). Now, how does one determine the amount of nitrogen in food?
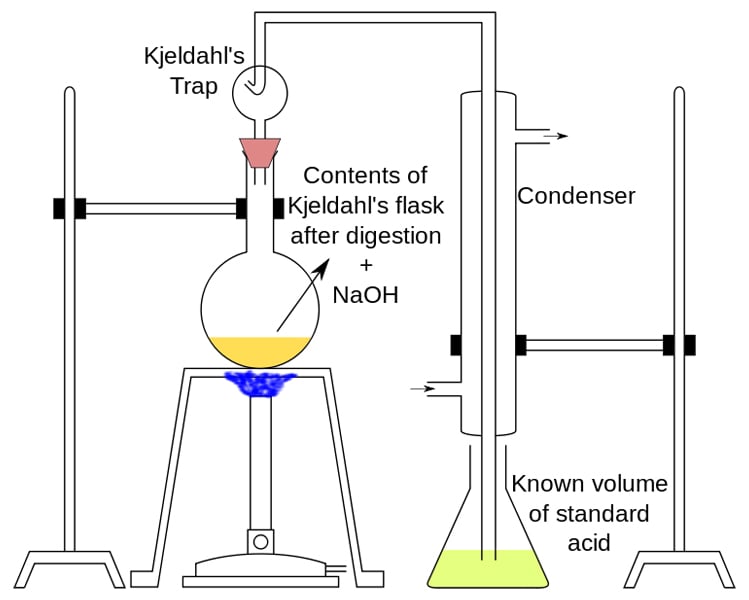
The classic methods used for protein (nitrogen) determination are the Kjeldahl method and the Dumas method. AOAC International has adopted the Kjeldahl method, so it is the method used by most food standards agencies. However, the Dumas method has also been approved by other standards organizations.
The Kjeldahl method makes use of Sulphuric Acid (H2SO4) to decompose a given food sample. This releases nitrogen from the food as ammonium sulfate (NH4) 2SO4. The amount of ammonia released is measured and the amount of nitrogen is subsequently determined. Multiplying the amount of nitrogen by 6.25 gets us the protein content in the food sample. The actual method is slightly more complex and deserves an article of its own.
Fats
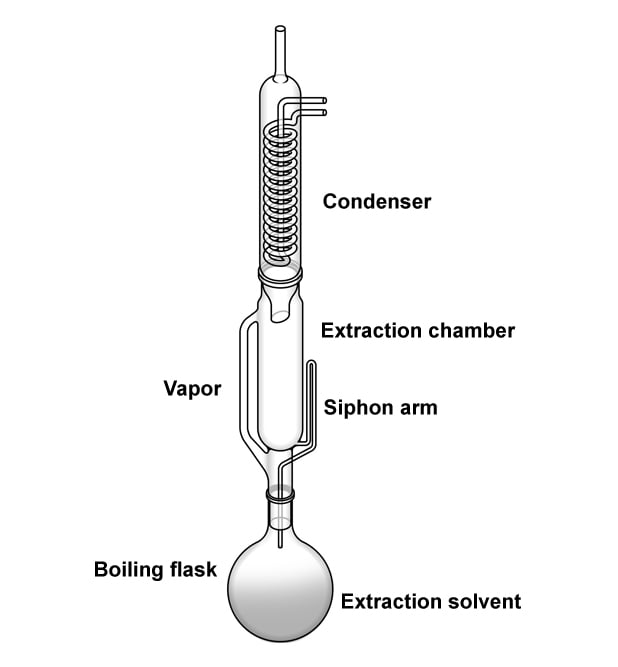
The most important nutrient that people try to keep in check are fats. Also, fats are the easiest of the three macro-nutrients to scale. They are insoluble in water and soluble in organic solvents, such as ether and chloroform. We put this property to use when measuring the quantity of fats in food. The methods used to measure fats are solvent extraction, non-solvent extraction, and a few other instrumental methods. Solvent extraction methods are the most commonly used and are the officially recognized methods to determine a food’s fat content.
The food item is first weighed and then placed inside a solution of ether (or hexane). Ether, being an organic solvent, washes away/dissolves the fats. The food is then weighed again. The difference in the sample’s weight before and after treatment with an organic solvent provides the amount of fats present in the food. The actual process is divided into four steps: drying the sample, particle size reduction, acid hydrolysis, and the selection of solvent. Commonly used organic solvents are ethyl ether, petroleum ether, pentane, and hexane.
Non-solvent extraction methods make use of other chemicals aside from organic solvents. These methods are mainly employed to measure the fat content in milk and other dairy products. These methods are the Babcock method, the Gerber method, and the detergent method. Both solvent and non-solvent extraction methods have their respective drawbacks. These methods require appropriate sample preparation, expert supervision, are time-consuming, destructive and not precise.
On the other hand, instrumental methods like Nuclear Magnetic Resonance and Ultrasonic or light scattering approaches are simple and give precise results. They are rapid, require little sample preparation and are non-destructive. However, the instruments are expensive and cannot be used for all types of foods.
Carbohydrates
Carbohydrates are the body’s main source of energy and make up almost 70% of our caloric intake. Now, if carbs are the primary source of energy, then why do they get such a bad rep? Well, not all carbs are created equal. Carbohydrates can be divided into simple carbohydrates and complex carbohydrates. The two types of carbohydrates can be further branched into sugars, fibers and starch.
Simple carbohydrates are simple sugars, i.e., they contain 1 or 2 molecules of sugar. We further divide these into single sugars (monosaccharides) and double sugars (disaccharides). Examples of simple carbohydrates are table sugar, candy, honey, fruits, etc. Simple sugars are the quickest source of energy and are easy to digest. Simple carbs aren’t something that you should eat in excess (except fruits) and are the carbs responsible for the bad reputation. Complex carbs are made of multiple sugar molecules and therefore take longer to digest. Examples of these include oats, whole grains, greens, lentils, etc. Starch and dietary fibers are also complex carbohydrates.

Carbohydrates are measured indirectly, rather than using experimental methods. First, the amount of other nutrients in the food (protein, fats, water, ash, and alcohol) are determined individually. We then subtract the sum of these individual values from the total weight of the food. Thus:
100 – (weight in grams of [protein + fat + water + ash + alcohol] in 100 g of food) = Total Amount of Carbohydrates
It should be noted that this amount includes all kinds of carbs present inside the food. Dietary Fiber, a carb, is not digested in the human body. To determine the amount of available or useful carbs, we need to subtract the amount of fiber from the quantity of total carbs in each food item.
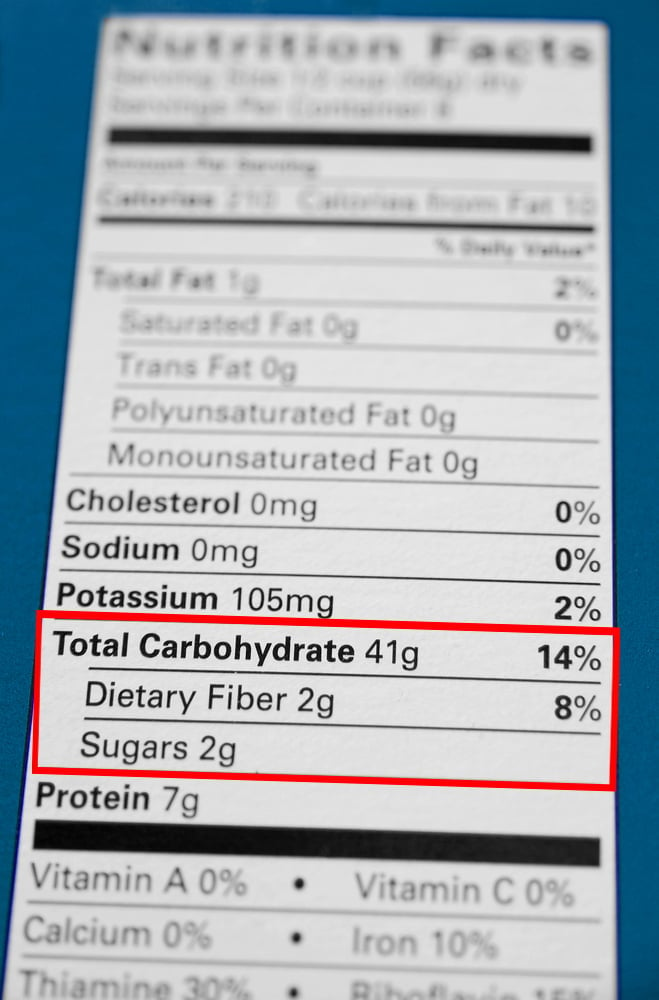
Dietary Fiber is measured using the enzymatic-gravimetric method. After preparing the food sample, it is treated with enzymes that mimic the digestive process in the human small intestine. Digested nutrients are removed from the sample using precipitation and filtration. Whatever remains is dietary fiber, proteins, and some other inorganic material. The remaining sample is weighed and the number of proteins and inorganic stuff (measured beforehand using analytical methods) are subtracted from it. The final number represents the amount of dietary fiber in the food.
However, a slight error in the calculation of any other nutrient can lead to inaccurate values. Thus, experimental methods, such as Thin-Layer Chromatography (TLC), High-Performance Liquid chromatography (HPLC), chemical-based methods like titration, gravimetric, colorimetric, and physical methods like polarimetry are also used.
Total Number Of Calories
Let’s start by first defining a calorie. A calorie is a unit of energy. The scientific definition of a calorie is the amount of energy required to raise the temperature of 1 gram of water by 1 degree celsius. Some other common units of energy you might know are joules, watts, and horsepower. In reality, the Calorie that we see mentioned on food labels is actually a kilocalorie. So 1 food Calorie (kcal) is equal to 1000 calories. Now, how do manufacturers calculate the total number of calories in your food?
Traditional Method
The traditional method used to measure the total amount of Calories in a food item involves using a bomb calorimeter. The food is placed inside a sealed container filled with water. The food item is then burnt using electrical energy. Once it has completely burned, the rise in the temperature of the water is measured. The rise in the temperature of the water is equal to the energy/Calories in the food.
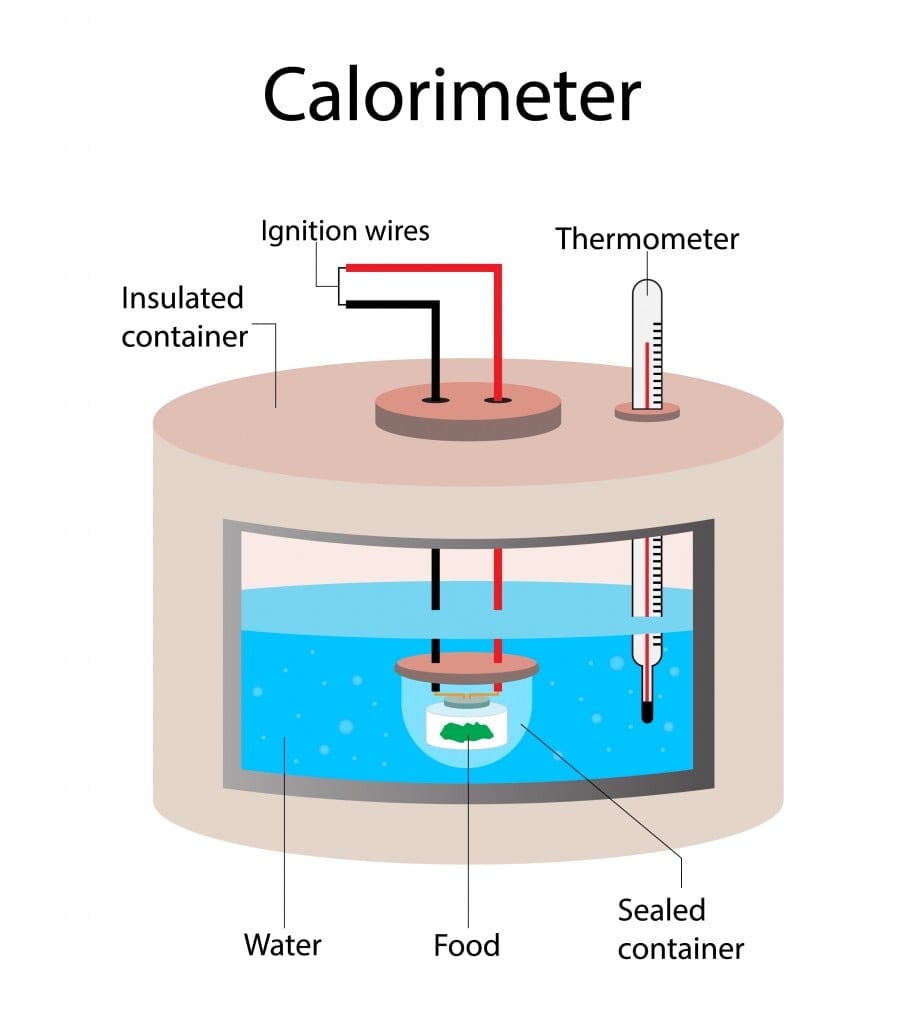
However, this technique is no longer practiced for measuring Calories. The bomb calorimeter measures all available Calories, which includes Calories from indigestible nutrients like fiber. Thus, bomb calorimeters resulted in a constant overestimation of Calories available to us in food items. This method also—along with burning food—burned a hole in manufacturers’ pockets.
Modern Methods
Under the Nutrition Labeling and Education Act of 1990, the FDA began demanding that food manufacturers specify the amounts of nutrients and Calories in their products. Since the bomb calorimeter method was expensive, a simpler and much more accessible method was adopted. This new method is the Atwater system, in which the total number of Calories is found by summing up the Calories stored in each energy-containing nutrient. This includes proteins, carbohydrates, fats, organic acids and alcohol. The values for each nutrient are 4 Kcal/g for protein, 4 Kcal/g for carbohydrate, 9 Kcal/g for fat, 3 Kcal/g for organic acids and 7 Kcal/g for alcohol.
For example, the label on a chocolate bar with 10g protein, 15g carbohydrate, and 30g fat would have a total Calorie count of 370 kcals.
Conclusion
The methods discussed above are the traditional methods and require laboratory analysis. However, because of developments in technology, food manufacturers are no longer required to follow such cumbersome procedures. The availability of online databases and nutritional analysis services has made the preparation of nutritional charts much easier. One can provide details like the ingredients used, recipe, cooking method, serving size, etc. and have a nutritional chart easily made up.
You might be wondering, are these nutritional chart values verified by some agency? Is it possible for manufacturers to mislead consumers about the nutrients present in their food? Casey Neistat, a New-York based Youtuber, took to the streets of New York to find out ‘The Truth Behind Calorie Labels’.
References (click to expand)
- Mæhre, H., Dalheim, L., Edvinsen, G., Elvevoll, E., & Jensen, I.-J. (2018, January 1). Protein Determination—Method Matters. Foods. MDPI AG.
- ANALYSIS OF LIPIDS. The University of Massachusetts Amherst
- CHAPTER 2: METHODS OF FOOD ANALYSIS. The Food and Agriculture Organization of the United Nations
- How To Read Food and Beverage Labels. The National Institute on Aging
- Nielsen S. S. (2017). Food Analysis. Springer International Publishing
- Nutritional Labeling and Education Act (NLEA) Requirements (8/94 - 2/95) | FDA - www.fda.gov