Table of Contents (click to expand)
Salt helps to preserve food by drawing out water and by inhibiting the growth of bacteria.
‘Don’t be salty’ or ‘why so salty?’ isn’t a great comment to receive, but if you happen to be raw meat in a storage unit, waiting to be picked up for someone’s dinner, being salty may be the best thing for you! You probably already know that salt is used to preserve food items like meat, fish and pickles, and are curious about the answers to some other questions. For example… Why is salt used for preservation purposes? How does salt help in the preservation of foods? For those answers and more, read on!
Recommended Video for you:
A Brief History Of Salt
Apart from being used to add flavor to food, salt has also played an integral role in world history. Let’s take a trip down memory lane, shall we?
The Egyptians used salt as a form of religious offering to their Gods, while the Phoenicians used salt as a valuable trade item in the Mediterranean empire. Even the Romans valued salt highly and used it as a form of currency. Even the Holy Bible contains references to salt. The modern words ‘Salary’ and ‘Salad’ have their origins in the word ‘salt’ as well.
Among all of its various uses, people from medieval times found another very important use of salt—to preserve their precious food! This newfound use added to the value and importance of this white granular substance that we now find on every dinner table.

How Does Salt Help In The Preservation Of Food Items?
As we already mentioned, the method of adding salt to foods in order to preserve them has been around for centuries. Salting helps to increase the shelf life of certain food items and keeps them safe for future consumption. There are two primary ways in which salt helps in the preservation of food. It does this firstly by drawing out moisture from the food, and also by inhibiting the growth of bacteria and other micro-organisms.
Drawing Out Water (Dehydration)
Osmosis… do you remember that term from your school days?
At some point in our school lives, we all performed the “raisins in water”experiment, right?
A bowl was filled with water and raisins were soaked in the bowl for a few hours. Later in the day, the raisins had swollen up as water moved from the bowl to inside the raisins through their skin. That was the whole experiment. This process of water moving from a location of high concentration to a location of low concentration through a semi-permeable membrane (the skin of the raisins in this experiment) is called osmosis.
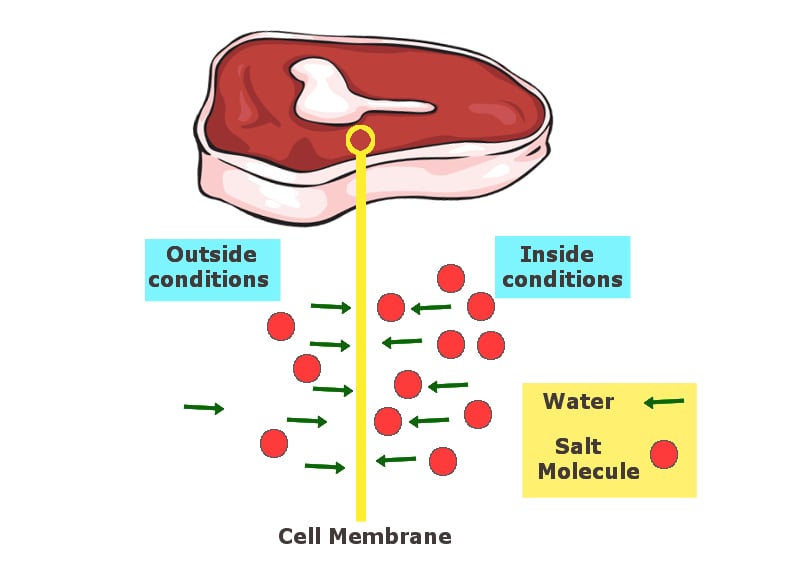
Similarly, salt draws out water from food items and dehydrates them. The salt, either used in solid or liquid form (brine), has the tendency to reach equilibrium with the salt content of the food with which it is in contact. This draws out water from the inside of the food and replaces it with salt. In the absence of water/moisture in the food, food-borne pathogens like salmonella and other bacteria cannot survive. These are the same bacteria that cause food poisoning, along with other serious food-related issues
Inhibiting The Growth Of Bacteria
Dehydrating the food items alone makes the bacteria’s chance of survival quite bleak. Furthermore, using salt for this purpose makes it even more difficult for the microbes to grow. Salt, chemically known as Sodium Chloride, affects different organisms in different ways.
Based on how micro-organisms react to sodium chloride, they are divided into two categories: Halophiles and Non-Halophiles. ‘Halo’ stands for salt and ‘phile’ for loving. Both words combine to form ‘Salt-Loving’.
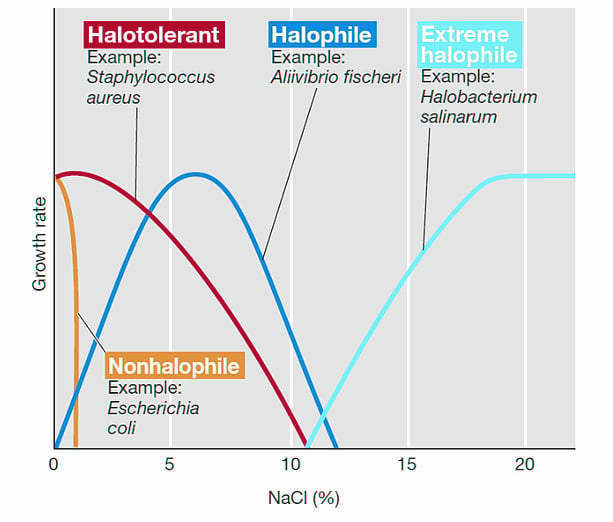
Halophiles are further divided into three types: slight, medium and extreme, based on their affection/tolerance for sodium chloride. While halophiles love salt, the same environment can be deadly for non-halophiles. Adding salt to a culture of non-halophiles inhibits them from growing. This is also a technique often used by scientists when they want to inhibit the growth of a certain culture medium of non-halophiles. The technique is called ‘selective medium’.
Osmotic Shock
Adding salt to foods causes the microbial cells to experience osmotic shock. This osmotic shock causes them to lose water from cells, resulting in cell death or retarded growth. Some studies also suggest that sodium chloride may limit oxygen solubility inside the microbial cells. It has also been known to interfere with cellular enzymes and forces cells to expend energy to throw out sodium ions from the cell. All of this results in the growth inhibition of the bacterias and ultimately causes their death.
Conclusion
These are the two ways in which salt helps to increase the shelf life of various foods, particularly our beloved meat. However, some modern methods like refrigeration have led to a decline in the use of salt as a preservative substance. The major reason for using salt for preservation purposes is because it is non-toxic, inexpensive and easily available. And obviously, because it makes everything taste better!