Table of Contents (click to expand)
The main difference between soaps, shampoos and detergents is their intended purpose. Soaps are made with animal fats or vegetable oils and caustic soda and are intended for cleaning the body. Shampoos are made with a surfactant and a co-surfactant and are intended for cleaning the hair. Detergents are made with a variety of chemicals and surfactants and are intended for cleaning clothes, dishes, or other surfaces.
Back in the days of our ancestors – who lived as hunter-gatherers and had to toil to get something to eat – I believe that people paid less attention to how they washed their hair or hands than they did to avoiding getting eaten alive by a wild beast.
However, the people of the 21st century are spoilt for choices when it comes to choosing the best product for personal hygiene. You have soaps to bathe with, shampoos to clean your hair, detergents to wash your clothes with and so on. Yet if all of these products are essentially soaps, what’s the difference between them?

In order to answer that, we’d have to look at the technical definition of a soap.
Recommended Video for you:
What Is A Soap?
We all know and identify a soap as something that helps us wash stuff, but what actually is a soap? What is its chemical definition?
A soap, in essence, is the salt of a fatty acid. A soap molecule consists of a polar ionic hydrophilic end (“water-loving’ end) and a nonpolar, hydrophobic end (“water-hating” end). When soap is mixed with water, its molecules arrange themselves in the form of roughly spherical aggregates of 60 or so molecules.
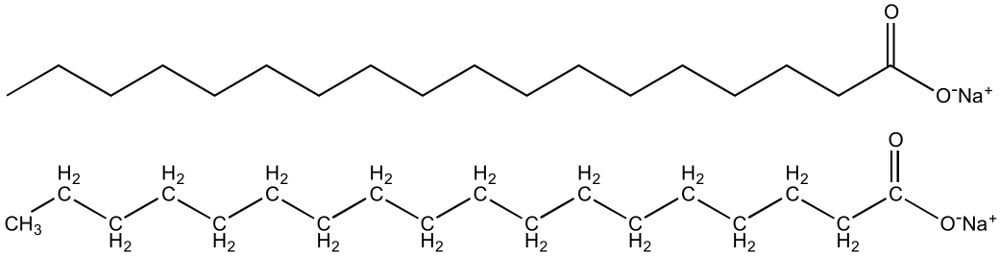
‘True’ soaps are produced by mixing animal fats or vegetable fats with a strongly alkaline solution, such as lye (usually sodium hydroxide) or potash (fertilizer potassium). As such, from a purely technical standpoint, most modern liquid soaps are not actually soaps.
Different Types Of Soaps
Technically speaking, most modern soaps are not really soaps. They are usually mixtures of petroleum-derived surfactants (e.g., sodium lauryl sulfate) with other chemicals that produce detergents matching the desired use. Depending on the type of chemicals you use, you can get very different types of soaps. For instance, using potash will yield a liquid soap, while lye will form a hard soap, like what you get in the shape of bars.
The other factor that matters is the fat you use while making these soaps; olive oil makes a very soft soap, while lye makes a very hard and dry bar of soap.

It must be noted that all detergents – regardless of what they’re used on (e.g., hair, hands, clothes, dishes etc.) work on the same basic principle: they break up oils and dirt and wash them away with the help of water. Thus, as far as their general purpose is concerned, all soaps are technically pretty much the same.
However, it’s also true that different products are suited for different conditions, and therefore cannot be used interchangeably. For instance, you wouldn’t want to wash your hair with the soap that you use in your washing machine, would you?
 Below are a few common types and forms of soaps:
Below are a few common types and forms of soaps:
Shampoo
A shampoo is usually made by combining a surfactant (generally sodium lauryl sulfate or sodium laureth sulfate) with a co-surfactant (usually cocamidopropyl betaine) in water to form a thick, viscous liquid. It’s designed to be gentle on keratin (a fibrous protein important for hair formation).
Shampoo is also able to rinse out from the hair quickly, thanks to its fairly low concentration of surfactants.
Hand And Body Wash Soaps
These soaps are typically formed with a mild surfactant to avoid irritation of the skin, and they have a bunch of other ingredients that have a few added benefits, such as adding a pleasant smell, moisturizing the skin, improving lather etc.

Hand washes are also typically less foamy, since they don’t need to cover very much surface area and are not used as frequently. These soaps are also more concentrated than shampoo. Face washes have even more specialized ingredients, such as ceramides and multivesicular emulsions, alpha hydroxy acids, benzoyl peroxide or salicylic acid, etc. which, in addition to keeping the face clean, also help to fight conditions like acne.
Bar Soaps
Bar soaps are the closest thing we have to the ‘true’ kind of soap, i.e., they are formed with animal fats or plant oils and caustic soda (sodium hydroxide, which is a strong alkaline substance). In addition to the basic ingredients, which remain pretty consistent in all bar soaps, they may have added ingredients to enhance the color, texture and scent.

Laundry Detergents
Laundry detergents are highly concentrated (unlike shampoos and body wash soaps), as they are used only after getting diluted by large amounts of ‘wash water’. That’s why it’s such a task to rinse off even a few drops of liquid detergent on your hands (because they are so concentrated).
They contain certain surfactants that can work with both cold or hard water (as these surfactants are less susceptible to water hardness), which is why they are much better at washing clothes than your run-of-the-mill bar soap.

Traditional washing powders are usually of a higher pH than liquid detergents, which are closer to a neutral pH.
Dishwashing Detergents
Dishwashing detergents that are used with hands directly must remove dirt, grease, sugar etc. from dishes without being too harsh on the skin. That’s why they don’t contain bleach, and instead use a mixture of surfactants that work near neutral pH and are mild on the skin.
Dishwashing detergents used in automatic dishwashers, on the other hand, don’t care about being gently towards your skin, as they don’t usually come in direct contact with your skin or any organic fibers. As such, these detergents can and do use much harsher ingredients, which often include abrasives. In short, they rely on a brute-force approach by breaking down food and stains with high pH and chlorine bleach, but would be quite harsh to the skin if they came in contact with your hands.













