Table of Contents (click to expand)
Glass is an insulator, so, when glass experiences rapid changes in temperature, one side of it shrinks faster than the other, leading it to crack.
A few days ago, I placed a glass bottle of water in the freezer so the water would cool quickly. As expected, I promptly forgot that I did so. When I opened the freezer the next day, I discovered that the water had turned to ice (an expected outcome) and the bottle and been broken (unexpected outcome)! Thus, the question of my day… why did the glass break?
To answer this, we need to understand thermal equilibrium and glass as a conductor of heat. So… let’s dive in!
Recommended Video for you:
Thermal Equilibrium
Thermal energy is the energy tied to the temperature of an object or system. When talking about heat, we are referring to the transfer of thermal energy from one object to another. Heat is transferred either through conduction, convection or radiation. In general terms, the laws concerned with the exchange (and change from one form to another) of thermal energy are called the Laws of Thermodynamics. These laws govern our understanding of changes in temperature in any system.
Zeroth Law Of Thermodynamics
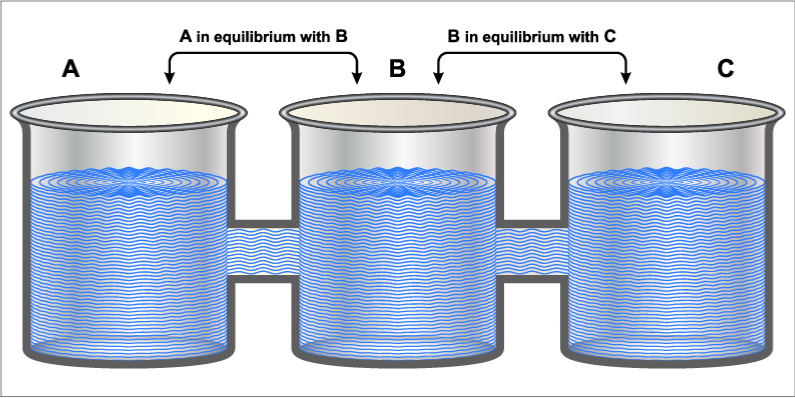
In the light of our question, the law that is most relevant is the Zeroth Law of Thermodynamics. This law essentially states that “when two bodies have equality of temperature with a third body, then they have equality of temperature”. This implies that when systems at different temperatures are in contact, they will reach a point of thermal equilibrium over time. This law is what forms the basis for thermometers! To understand this better, let’s define and better understand what thermal equilibrium is.
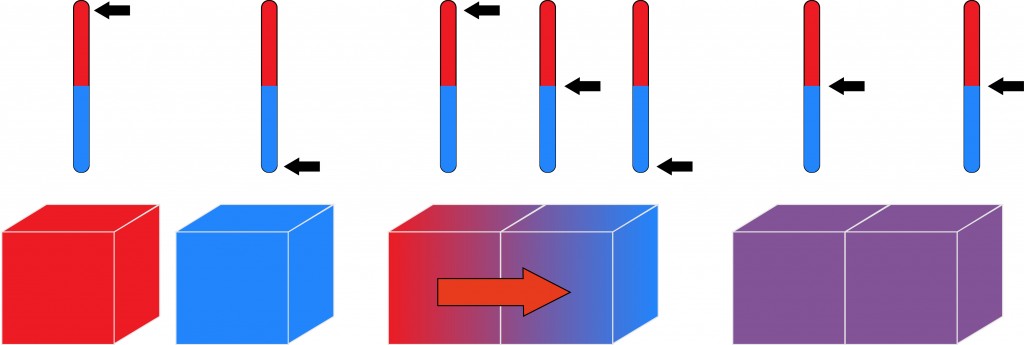
In the simplest of terms, when two bodies are in thermal equilibrium, they are in contact with each other and at are at equal temperatures. Therefore, if one of the bodies is hotter than the other, it will lose heat to the cooler body until the two are at an equal temperature.
How Does Glass Change Due To Changes In Temperature?
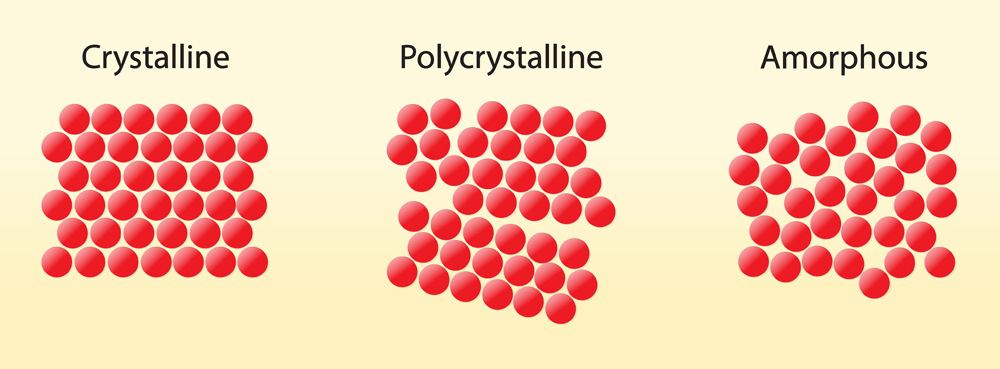
Solids have three kinds of structures; crystalline, polycrystalline or amorphous. An amorphous solid is a material which lacks a uniform order in its structure, A crystalline solid is perfectly ordered.
While a polycrystalline solid are those which have several different crystalline materials, in different orientations, comprised within the same solid. Glass is a ceramic material and is found in many different compositions of different amorphous solids. Glass is often largely made of silica. Silica exists in two kinds of states, crystalline and amorphous.
The kind of silica used in glassmaking is the amorphous or non-crystalline kind. The most common kind of glass is the optically transparent ceramic made of amorphous silica or silicon dioxide (SiO2) and an added approximately 30% (source) of sodium oxide (NaO), lime (CaO) and magnesia (MgO). As an object is supplied with heat, it will expand in volume, whereas it will shrink when it loses heat. Glass also reacts in this manner when it undergoes changes in temperature.
Heat, like electricity, has both good and bad conductors. Glass is a very bad conductor of heat. This property is due to its amorphous structure. The disordered atoms of the ceramic are unable to reliably transfer thermal energy. Because of its insulating properties, a thicker piece (like that of a bottle) is enough to cause a discrepancy in size if the temperatures are different on each side. Due to glass being a bad conductor of heat, the two sides will take longer to reach a state of thermal equilibrium.
Why Does The Glass Break?

Glass objects, due to these thermodynamic properties, are susceptible to breaking when exposed to rapidly changing temperatures. Heat must be exchanged through a medium.
In our question, the two objects are chilled air and the glass itself. The cold air acts as the medium through which heat is lost. The freezing air comes in contact with one side of the glass first, and that side of the glass rapidly loses heat, consequentially shrinking. The contrast in temperature exists because the two sides of glass are unable to reach a state of thermal equilibrium with the freezing air, as we discussed earlier.
Thus, when the heat is lost from one side of the glass, the other side is comparably warmer and therefore, more expanded. This contrast in the expansion then causes the glass to crack or break.












