Table of Contents (click to expand)
Paper is made of fibers that are tangled together. When water is introduced, the fibers start to separate from each other, making the paper very weak.
Gather a bunch of used papers, pile them up and try to tear the stack in half. If you’re not an unusually powerful fellow, it’s highly unlikely that you could tear it. Now, put that same stack in a bucket full of water and let it soak for a while. Take it out a few minutes later and try to tear it again. You should be able to tear it very easily… why is that?
Why does paper become so incredibly weak when it’s wet?
Short answer: Paper is composed of fibers that are tangled together; they start to separate from each other when water is introduced.
Recommended Video for you:
What Is Paper Made Of?
You must have heard many times that we should conserve paper because “trees are cut down to make them”. Well, that’s exactly right. In essence, a piece of paper is just wood.

Wood is actually a heterogeneous substance; 40-50% of it is micro-fibrils of cellulose and 15-25% is hemicellulose, both of which are held together by a natural glue called ‘lignin’. (Source) To make paper out of wood, you first need to turn raw wood into ‘pulp’ – a watery soup of cellulose fibers, water, lignin and certain other chemicals used during the process. After the pulp is ready, it goes through several additional processes to ultimately take the form of paper as we know it.
Cellulose Structure
You might have read or heard the term ‘cellulose’ many times in discussions pertaining to cotton, wood, dried hemp and other similar materials. It is the most abundant organic polymer (an organic substance whose molecular structure consists of a large number of similar units bonded together) on the planet. It is the chemical structure of cellulose, which happens to be the most important constituent of paper, that keeps the paper ‘rigid’ when dry and extremely fragile when wet.
Take a look at the structural formula of cellulose:
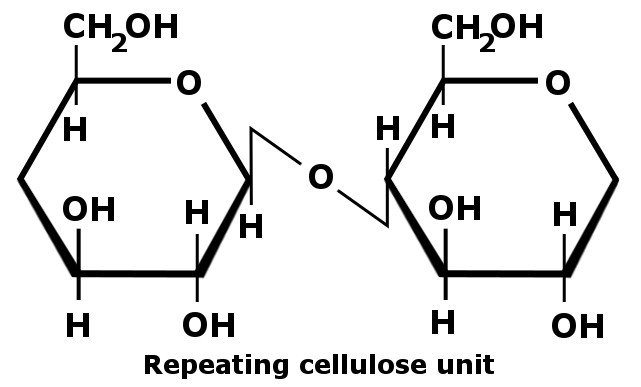
The numerous hydroxyl groups (-OH) present in cellulose form hydrogen bonds with oxygen atoms on the same chain or a neighboring chain. Also, one thing you should know about hydrogen bonds – they are incredibly strong! They help hold the long chains of cellulose together and impart that ‘sturdiness’ to paper.
What Happens When Paper Gets Wet?
Introducing water to paper brings about a drastic change in its ability to ‘stand up straight’, which severely comprises its strength. You see, cellulose is hydrophilic (Source), meaning that it has an affinity towards water and tends to dissolve in it. When water is added to paper, the hydrogen bonds holding the cellulose fibers begin to break down. This is because water molecules consist of oxygen and hydrogen atoms, which form hydrogen bonds with cellulose fibers, thus weakening their own hydrogen bonds in the process.

In short, you could say that adding water leads to the weakening of the fiber-fiber bonds of paper and leads to an unsteady equilibrium of fiber-fiber and fiber-water.
Wetting paper has a dramatic influence on the strength of paper, such that you could easily tear huge stacks of them apart with little effort. In fact, paper becomes so weak that it can disintegrate all by itself, which can be pretty bad in some cases.

We all know that wet paper is incredibly weak, so it should be handled with the utmost care. Still, now that you are equipped with a bit of scientific insight into how the disintegration of wet paper actually works, perhaps you’ll check your pockets twice before doing the laundry!