Table of Contents (click to expand)
The answer to this is both yes and no! The scene is a good blend of both fact and fiction.
If you’re a science geek or TV buff, you have to admit that Breaking Bad is as good as it gets—a perfect examples of an amazing union of entertainment and science.

Recommended Video for you:
About Breaking Bad
The show traces the life of a high school chemistry teacher turned drug kingpin named Walter White, who, upon being diagnosed with stage-three lung cancer, leaves his seemingly complacent and unrewarding life behind to produce the purest methamphetamine the streets of Albuquerque, New Mexico, has ever seen.
Pairing up with his former high school student and now delinquent, Jesse Pinkman, a small-time meth dealer and distributor, Walt paved his pathway from a simple educator to the infamous Heisenberg.

Based on some mind-boggling science facts, this show taught a lot of cool chemistry stuff to viewers around the world.
Unlike other crime lords who would get knocked out quick without a gun in hand, Walter could always conjure up something in a jiffy, ensuring that no one knew what hit them.
But how much of what we saw was actually fact and not fiction?
For the sake of caution, I should add a disclaimer NOT to take this as a sign to try any of this out at home. Just sit back and marvel at the scenes happening on the TV screen instead.

But How Real Was The Show?
Apart from piquing curiosity among kids and adults alike, the science behind the show is astonishingly factually correct and, in most cases, practical. Nonetheless, in some ways, it falls short of reality. One such example is the makeshift battery Walt and Jesse make in the episode “4 days out”.
Before getting access to the meth super lab in the seasons ahead, Walt and Jesse had a mobile meth lab—an RV—that they took far out in the desert for their wildly successful cooks. Their plan worked out magnificently for them, with bundles of cash piling up, until the end of one such cook, when they find that Jesse left the keys in the ignition for a couple days, leaving the RV’s battery dead, stranding them in the desert with no cellphone reception or access to the outside world.
Walt (after his initial meltdowns and routine bashing of Jesse) comes up with the ingenious plan to build a mercury battery to jump-start the RV.

Structure Of A Battery
Looking at what a battery is generally composed of, the basic functional parts include:
- Anode: the negative electrode that holds charged ions and gives up electrons;
- Cathode: the positive electrode that contains the discharged ions and absorbs electrons;
- Electrolyte: the solution that allows ions to move from the anode to the cathode, generally composed of salt water or acid;
- Conductor: it carries the electrons and is usually a metal wire
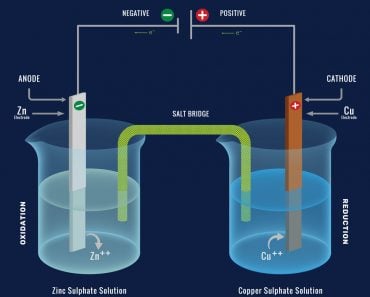
A battery both generates and stores electrical energy through chemical reactions. Suppose you put two different metals (anode and cathode) in an acid or electrolyte solution.
In such a case, the difference in the chemical reactivities of the metals produces a flow of current between them, which forms a basic electrochemical cell. A series of these cells is put together to form a battery.
Walter White’s Version Of The Battery
Following the basic design, this is how Walter made his battery:
- He found coins, nuts, bolts, and any other pieces of metal from the RV that contained zinc for the anode, where the electron loss would happen;
- He took the RV’s brake pads, made of mercuric oxide and graphite, to make the cathode;
- The leftover potassium hydroxide from the meth-making was the electrolyte;
- The copper wire was the conductor.
He separated the anode and cathode with sponges absorbing the electrolyte (if the anode and cathode touch each other, the current flow stops). He put six of these fuel cells together, and voila! The RV trudged them away from their near-fatal cookout.
However, despite the solid structural design, such a simple battery would only provide a fraction of the power actually required to turn over an engine. It’s highly unlikely that this battery would have actually ever worked in reality, but it did make for a great episode!

The show is quite accurate in most of its scientific endeavors, making it a fascinating blend of reality mixed with a little imagination. Fanning the flame of curiosity in young science enthusiasts, Walter White will teach you to “simply respect the chemistry”, and he’s not a man with whom you want to argue!