The body cannot digest fibers because it doesn’t have the required enzymes to do so.
A major part of our diet consists of sugars, also known as carbohydrates. They are an important macronutrient that is readily available as fuel for the cells. However, there are only certain types of carbohydrates that our bodies can digest. While we can enjoy and digest the sweetness of glucose, sucrose and starch, we can’t digest fibers from plants.
So, what makes fibers impossible for our bodies to digest?
Recommended Video for you:
Structure And Functions Of Different Carbohydrates
The structure of fiber is key to understanding why our guts can’t digest it. Fibers are polysaccharides.
Carbohydrates can either be monosaccharides or polysaccharides.
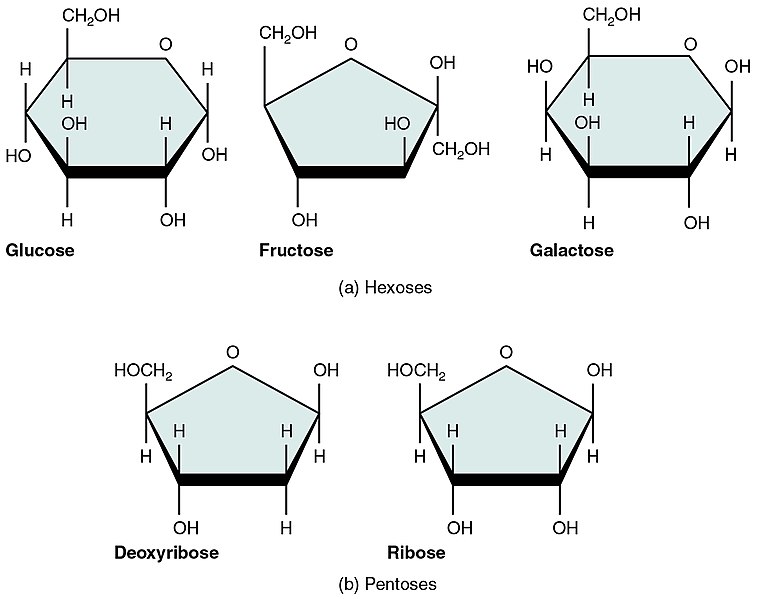
Monosaccharides are single-sugar molecules, such as glucose, one of the most commonly found sugars in nature, and the human body’s favorite sugar. Fructose is another simple monosaccharide abundantly found in fruits, and is similar to glucose. Monosaccharides can link to each other to form larger carbohydrates. Disaccharides contain two monosaccharides joined together. For example, sucrose (common kitchen sugar) is a disaccharide made by joining glucose and fructose.
When several monosaccharides link together, they form a polysaccharide. Polysaccharides can contain hundreds or thousands of monosaccharides connected to each other, making long chains. Some example of polysaccharides are starch components, such as amylose/amylopectin, cellulose and dietary fibers.
Given below is the structure of a common dietary carbohydrate.
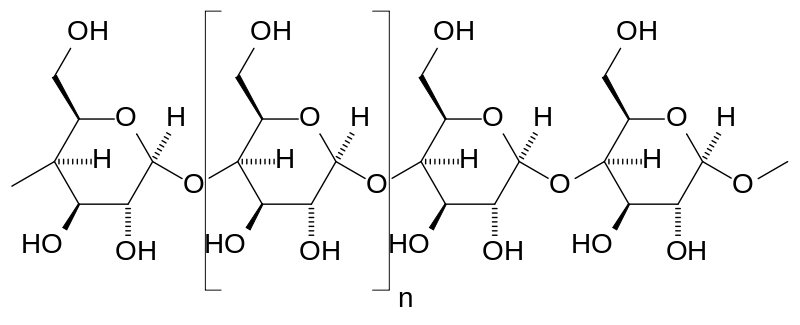
This is the structure of amylose—one of the components of starch—that is present in potatoes and bread (or anything starchy). The amylose polysaccharide is made of glucose molecules connected to each other.
These glucose linkages differentiate digestible carbohydrates from dietary fibers.
Structure And Functions Of Fibers
Polysaccharides are often used for one of two things in nature: as a storage of energy-giving monosaccharides, like starch stores glucose, or as a strong unbreakable structural unit. The former is commonly starch or glycogen. The latter are usually fibers. Common fibers are cellulose, resistant dextrins, chitins and inulin (not to be confused with insulin, which is a hormone and not a fiber).
Shown below is a simplified structure of the fiber chitin, produced by fungi and insects.
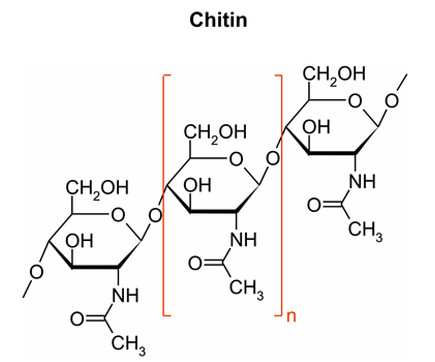
Dietary fibers, by definition, are indigestible by the human digestive system. Fibers characteristically have beta-glycosidic bonds between the component sugar molecules. These bonds make it indigestible by the body.
Digestion Of Alpha-glycosidic Bonds And Beta-glycosidic Bonds
Why does the type of bond make a difference in whether the body can digest one molecule over another?
Most carbohydrates that can be digested by our bodies are connected by carbons that have the same spatial arrangement. This is known as the alpha-glycosidic bond. The alpha-glycosidic bonds are present in disaccharides like sucrose and maltose, as well as polysaccharides, such as starch.
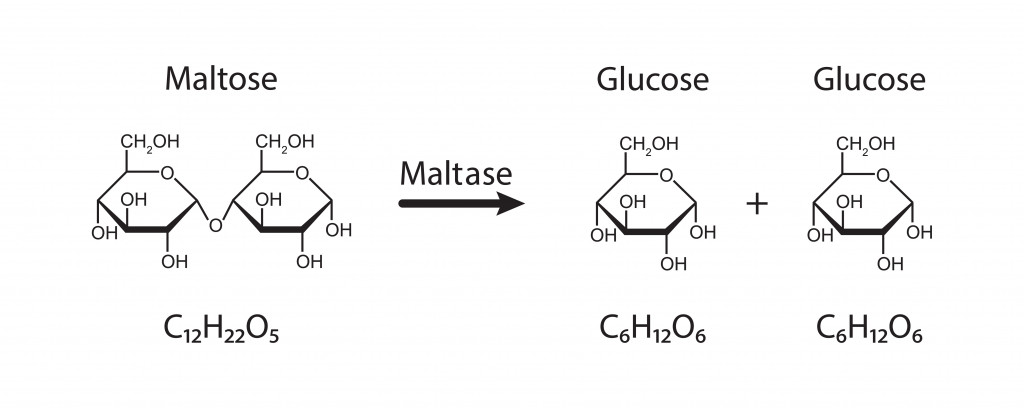
However, fibers have bonds known as beta-glycosidic bonds. In this, the carbons are arranged on the opposite sides, as shown in the figure below.
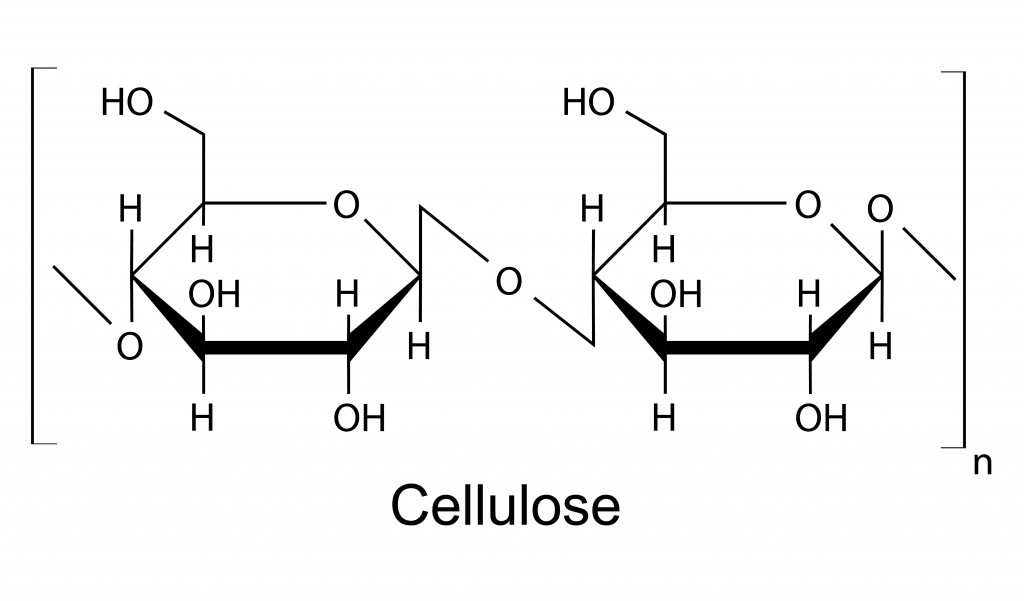
The carbohydrate-digesting enzymes in the body can only recognize alpha-glycosidic bonds. The human body does not have enzymes that recognize beta-glycosidic bonds. If the enzyme can’t recognize the molecule, it can’t break it down. This makes fibers indigestible by the body.
Biochemistry 0f Bonds For Nerds!
The difference in the glycosidic bonds is because not all sugar molecules have the same stereochemistry. Stereochemistry is the arrangement of atoms respective to each other. Even when the chemical formula of the sugars is the same, they can still exist in two forms that are structurally different from each other.
Moreover, every carbon that has four distinct groups for each bond is called a chiral carbon. The position of each group on the chiral carbon plays an important role in the biochemistry of the entire molecule. To understand it better, we take two forms of glucose, alpha-glucose and beta-glucose. These two molecules differ only in the positioning of one carbon group. However, it makes a huge difference in their structure and function in the body.
We saw how glycosidic bonds could either be alpha- or beta-glycosidic bonds. Alpha-glycosidic bonds form when carbon atoms on the two sugar molecules have the same stereochemistry. Hence, both the carbons have the alcohol group [-OH] on the same side, either facing upwards or downwards.
Beta-glycosidic bonds form when the carbons have opposite stereochemistry. One of the carbons has an alcohol group facing upwards, while the other has a downward-facing group. This leads to a more linear, and therefore stronger, structure to the molecule.
While this might look like a very minor change, it alters the chemical function of the molecule. Enzymes in the body are hyper-specific. Even a minute change in the stereochemistry means that the new molecule is unrecognizable to the enzyme.
Most of the carbohydrates found in nature have alpha-glycosidic linkages. However, fibers are known to have beta-glycosidic linkages.
Conclusion: Do We Need To Eat Fiber If We Cannot Digest It?
Fibers travel through the digestive system into the large intestine. In the large intestine, dietary fibers show water-holding capacity, adsorptive functions and cation-exchange. Cation exchange is the process of exchanging sodium, chloride and potassium ions to regulate the absorption of these salts. In addition, the exchange of these ions also helps in the efficient absorption of water from the remaining food excreta. In addition, dietary fibers also form mesh-like structures in the large intestine. These structures form the perfect micro-environment for healthy gut bacteria to survive and thrive.
These characteristics make fibers extremely beneficial for healthy bowel movements and excretion, despite our body’s inability to break them down!
References (click to expand)
- Carbohydrates - MSU chemistry. Michigan State University
- 1.4.1: Carbohydrates in the Diet - Chemistry LibreTexts. LibreTexts
- Turner, N. D., & Lupton, J. R. (2011, March). Dietary Fiber. Advances in Nutrition. Elsevier BV.
- (1982) Dietary fiber. - ScienceDirect.com. ScienceDirect
- 1,4 glycosidic bond - The School of Biomedical Sciences Wiki. Newcastle University