Table of Contents (click to expand)
- Metals In The Body
- I Am The Essence Of Your Food When Paired With Chlorine. Who Am I?
- When Paired With Oxygen, I Protect Your Skin From Tans And Sunburns. Who Am I?
- I Am The First Name Of A Superhero And Turn Rusty When Mixed With O2 And H2O. Who Am I?
- The Statue Of Liberty Is Made Of Me. I Patina When Corrosion Hits Me. Who Am I?
- I Am The Reason Why Rubies Are Red And Emeralds Are Green. Who Am I?
- I Have One Of The Highest Melting Points Of All Pure Elements, With Only Tantalum And Tungsten Surpassing Me. Who Am I?
- Conclusion
Metals such as sodium, potassium and magnesium (among many others) play a vital role in metabolic reactions, maintaining homeostasis (maintaining water balance), and passing electricity through the nervous system.
What do a house, a car, a lamp post and an automobile have in common? Answer: Metal.
But why is the same iron in your car so crucial for your blood? Why are metals, these shiny elements we can dig out of mountains, so important for our mostly carbon-, hydrogen- and oxygen-based bodies?
Recommended Video for you:
Metals In The Body
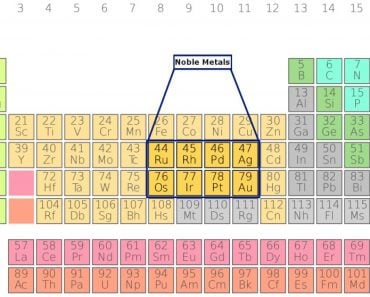
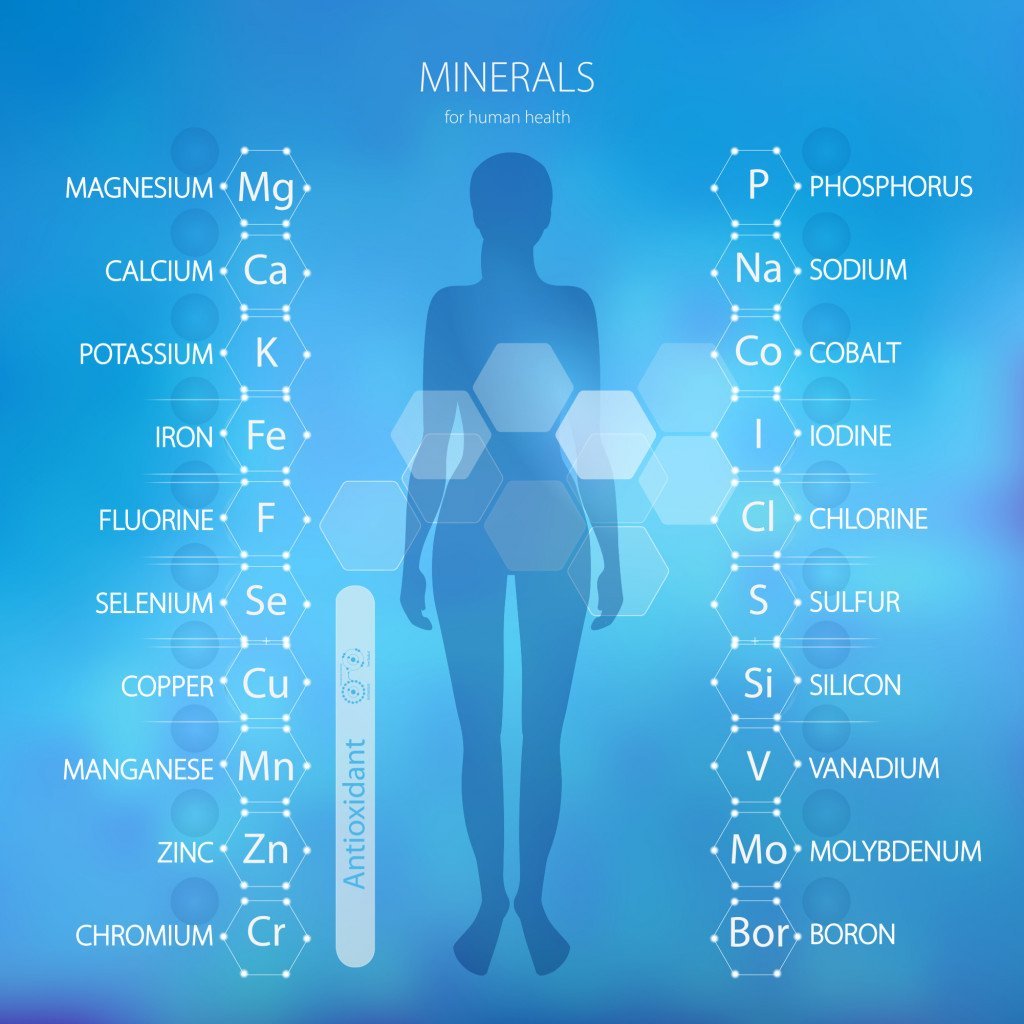
Metals are present in the form of ions in the human body. Metals that are essential for normal body functioning are:
Sodium (Na), Potassium (K), Magnesium (Mg), Calcium (Ca), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickle (Ni), Copper (Cu), Zinc (Zn) and Molybdenum (Mo).
However, some metals are toxic to the human body. Heavy metals are a group of relatively high-density metals that are toxic even at low concentrations. Elements like arsenic (As), mercury (Hg), lead (Pb), chromium (Cr) and cadmium (Cd) are considered heavy metals.
Let’s take a deeper look into the roles of some important metals in our bodies. To make things more interesting, you will have to guess the name of each metal with the help of some simple clues.
I Am The Essence Of Your Food When Paired With Chlorine. Who Am I?
Answer: Sodium.
Sodium (Na) is a soft silvery-white metal. About 85% of the sodium in the body is found in the blood and lymphatic fluid. It is necessary to maintain proper water balance within the cells to ensure the healthy function of nerve impulses and muscle function.
In partnership with potassium ions (K+), the sodium ions (Na+) act like a chemical battery that allows the buildup of electrostatic charge within the cells. Through a series of chain chemical reactions, these nerve impulses are carried to the brain or muscles for action. However, too much sodium can damage the kidneys and increase the risk of blood pressure.
When Paired With Oxygen, I Protect Your Skin From Tans And Sunburns. Who Am I?
Zinc is a trace mineral in the human body, meaning that we only require a small quantity to function normally. Yet, more than 300 enzymes and hormones depend on zinc to carry out important chemical reactions in the body.
Zinc helps in maintaining healthy immunity by supporting the growth and normal functioning of the immune cells. It aids in the production of new cells of collagen and fibre-like tissues that take part in wound healing. Furthermore, it is a much-needed mineral during times of rapid growth, i.e., during childhood, adolescence and pregnancy. What more, you ask? Well, due to its involvement in mitotic cell division and RNA and DNA repair, zinc plays a prominent role in aging.
Looking at the importance of zinc in the human body, it shouldn’t come as a surprise that zinc deficiency can be quite detrimental to our health. It can cause hormonal dysfunction, immunity impairment, delayed sexual maturation and growth retardation, just to name a few negative consequences.
I Am The First Name Of A Superhero And Turn Rusty When Mixed With O2 And H2O. Who Am I?
Iron, a lustrous greyish metal, is abundantly present in our red blood cells as hemoglobin, and is responsible for carrying oxygen-rich blood around the body. Other than keeping our cells alive, iron is also a key component of enzymes that synthesize steroid hormones, bile acid and certain neurotransmitters, such as dopamine and serotonin.
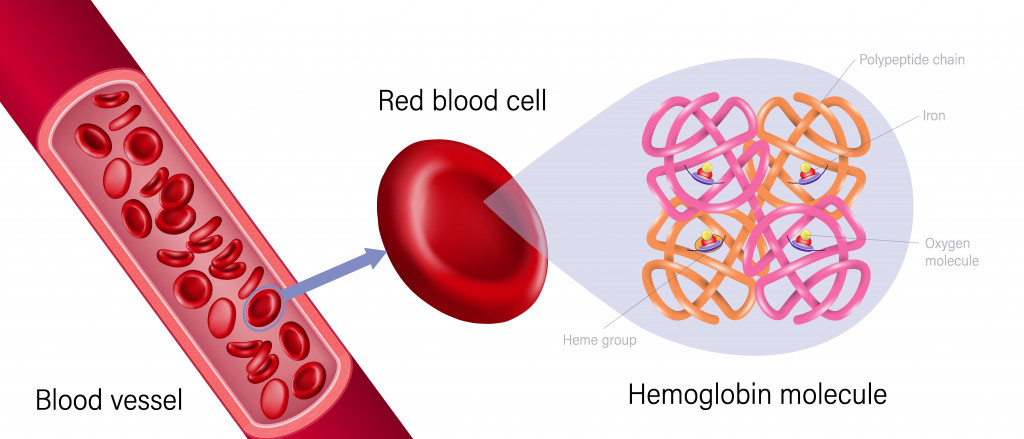
High concentrations of iron in the body can be fatal, as it may damage the liver and heart. On the other hand, a scarcity of iron in the blood can cause anemia, as the body requires iron for hemoglobin synthesis.
The Statue Of Liberty Is Made Of Me. I Patina When Corrosion Hits Me. Who Am I?
Copper, a red-orange metal, is an intrinsic part of the human body. Copper is a cofactor of many enzymes that are known as cuproenzymes. These enzymes help in energy production, the breakdown of iron, and neurotransmitter synthesis.
Copper also plays a key role in the development of new blood vessels, immune function, and brain development. It protects our cells from free radical damage to proteins, membrane lipids, and nucleic acids.
I Am The Reason Why Rubies Are Red And Emeralds Are Green. Who Am I?
Chromium—a heavy metal—is an essential mineral that the body requires in trace amounts. But wait, aren’t heavy metals toxic for the body? It so happens that trivalent chromium in small quantities doesn’t cause any harm to the body. However, hexavalent chromium is 100 times more toxic than trivalent chromium. This is because Cr (III) is not easily absorbed by the gastrointestinal tract, whereas Cr (VI) is absorbed by the lungs and the gastrointestinal tract.
Chromium is important, as it helps in the breakdown of carbohydrates, fats and proteins. Additionally, it aids in insulin action within the body.
I Have One Of The Highest Melting Points Of All Pure Elements, With Only Tantalum And Tungsten Surpassing Me. Who Am I?

Molybdenum, a silvery-white metal, is essential for the body, as it is an important part of several enzymes. Molybdenum-containing enzymes are responsible for breaking down certain drugs, toxins, sulfites and purines. Purines form uric acid when metabolized, whereas sulfites are preservatives used to prevent the discoloration of food and can cause an allergic reaction in sulfite-sensitive people.
Conclusion
Humans and all other living organisms flourish due to the presence of primary compounds like fats, carbohydrates and proteins. However, we need metals to actively function in conjugation with these foundational compounds. The body does need metals to function properly, but only within a specific range. No matter how crucial the metal is, if it exceeds the required amount, it will do more harm than good to the body. Conversely, a deficiency of any essential metal will hamper the normal growth and function of the body.
References (click to expand)
- Roles of metals in human health - MedCrave. medcraveonline.com
- Vitamins and Minerals | The Nutrition Source. The Harvard T.H. Chan School of Public Health
- The role of sodium in the body stantin1, Iliuţã Alexandru2. bioclima.ro
- CP Gupta. Role of Iron (Fe) in Body - Daf Yomi Review. iosrjournals.org
- What Are the Physiologic Effects of Chromium Exposure .... The Agency for Toxic Substances and Disease Registry
- Molybdenum - Health Encyclopedia - URMC. The University of Rochester Medical Center