Table of Contents (click to expand)
The main reason that life on Earth is carbon-based is because of the unique properties of the carbon atom. Carbon atoms can form strong bonds with other atoms, including other carbon atoms, which allows for the formation of complex molecules like DNA and proteins. Additionally, carbon atoms are relatively small, which makes them ideal for forming the millions of intricate chemical bonds and reactions that are necessary for sustaining life. Finally, carbon is abundant on Earth, making it the perfect element for building life from scratch.
Speculation about the existence of extraterrestrial life has been a perennial occupant of humanity’s endless capacity for wonder. To see if there is anyone out there, we sent both Voyagers, not to study a particular star, but to leave our shores and explore other islands in the vast oceans of outer space. Astrophysicists have also wondered (if we do discover one) about the nature of such an alien civilization.
If we were to infer the biochemistry of any life form from what we have already encountered, which is well, us, we’d conclude that it would be based on carbon. Carbon, or as science fiction regularly depicts, carbon’s closest cousin, silicon. However, Carl Sagan called this parochial view Carbon chauvinism. He believed that we shouldn’t limit our imagination and ridiculed the assumption that alien life would resemble life on Earth.

Still, due to its sheer dominance on Earth, carbon remains our best guess. Given that, what makes carbon so special?
Recommended Video for you:
Life Is Complex
Every life form, whether plant or animal, is the embodiment of complexity. The first self-replicating organism evolved more and more complex biological functions by incorporating more and more molecular complexity. Here, complexity refers to fostering millions of intricate chemical bonds and reactions that are imperative to sustain higher forms of life. A primitive life form, such as an amoeba, fosters far lesser molecular complexity than a higher mammal, such as a dog.
Molecular complexity enabled the synthesis of functions, such as breathing, excretion, digestion and most importantly, reproduction. None of this would have been possible without carbon. Without carbon, there would have been no DNA, proteins, lipids, sugar, fat, muscle tissue or anything else that makes up the stuff of life.

Considering the 118 elements known to man, it’s strange why only 5-6 of them are used to construct organic life. The most common of them is, of course, carbon, dedicated to whose antics is an entire branch of chemistry. It is carbon’s extraordinary thermodynamic and chemical properties that render it so superior to other elements.
Carbon Amiability
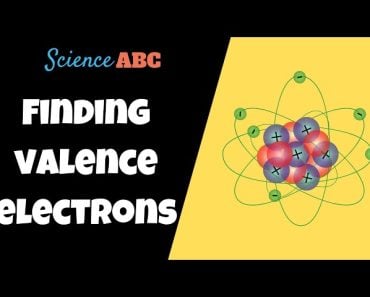
A carbon atom has four valence electrons, allowing it to form four single bonds (methane), two double bonds (carbon dioxide) and a triple bond (acetylene, a welding fuel and a raw material for synthesizing plastic). The dominance of carbon, however, isn’t a result of its ability to form these complex bonds, but rather the ease and pliancy with which it forms them. In fact, all the elements residing in the column that carbon occupies in the periodic table possess four valence electrons, but the stability of the bonds they form is incomparable to carbon.
Even silicon, the element that resides right below carbon, forms countless molecules, but a double-bonded silicon molecule, unlike double-bonded carbon, is transient – its instability eventually forces it to part into single-bonded silicon atoms. Carbon molecules, such as hydrocarbons, one of the most crucial species of molecules to sustain life, are neither too frail to easily break down, nor too rigid to deter plasticity and adaptability. This allows enzymes to easily manipulate carbon molecules. Furthermore, reactions with silicon aren’t all that efficient; silicon dioxide is a huge molecule (sand), as compared to carbon dioxide, which comfortably exists as a gas.
Silicon-based life can’t survive on Earth anyway. Silicon is more reactive than carbon and can form long ‘chains’ of molecules, reminiscent of hydrocarbons, but it will also react violently with oxygen at relatively low temperatures. This means that silicon chains or ‘silanes’ couldn’t have survived within our atmosphere. Carbon-based life also wouldn’t have survived if organisms stored energy directly as hydrocarbons — alkanes are quite flammable themselves (petrol and kerosene), but carbon-based organisms store energy as sugars, lipids, alcohols and other hydrocarbons that exhibit very different chemical properties.

All of these properties can be explained by a single statement: carbon is the smallest atom that possesses four valence electrons. Its size renders it the ideal friend to make. Bonds on one branch are unaffected by bonds on other branches. However, life would have been impossible to manufacture if nature lacked the raw materials itself. Carbon is abundant on Earth, so nature couldn’t have missed the opportunity. It leveraged its properties to build life right from scratch!