Table of Contents (click to expand)
Radiation is how the heat in a microwave oven is transferred. Microwaves are extremely adept at exciting and vibrating water molecules, and since food is mostly water, the vigorous motion of the molecules creates intermolecular friction, which generates the heat to cook the food item.
One day, after walking past a microwave radar he’d been working on, American engineer Percy Spencer found that the chocolate bar in his pocket had melted. Spencer realized that the intense microwave energy put out by magnetrons could not only be used to hunt enemies in war, but also cook pasta once in a while.
This discovery was revolutionary and purely accidental, a combination of adjectives we find irresistibly delightful. The first food item Spencer then deliberately cooked with microwaves – and I doubt this comes as a surprise – was popcorn. The second item he cooked without resting it on a flame, from a distance as though by sheer magic, was an egg, which exploded in the face of one of the experimenters. The moment was historic.

Raytheon, the firm that had employed Spencer, immediately filed a patent for a device that would cook or recook food by enclosing it and then bombarding it with microwaves. The device, with absolutely no regard for creativity, was simply called the microwave oven. But how does one work? Why did the chocolate bar melt?
Recommended Video for you:
Radiation
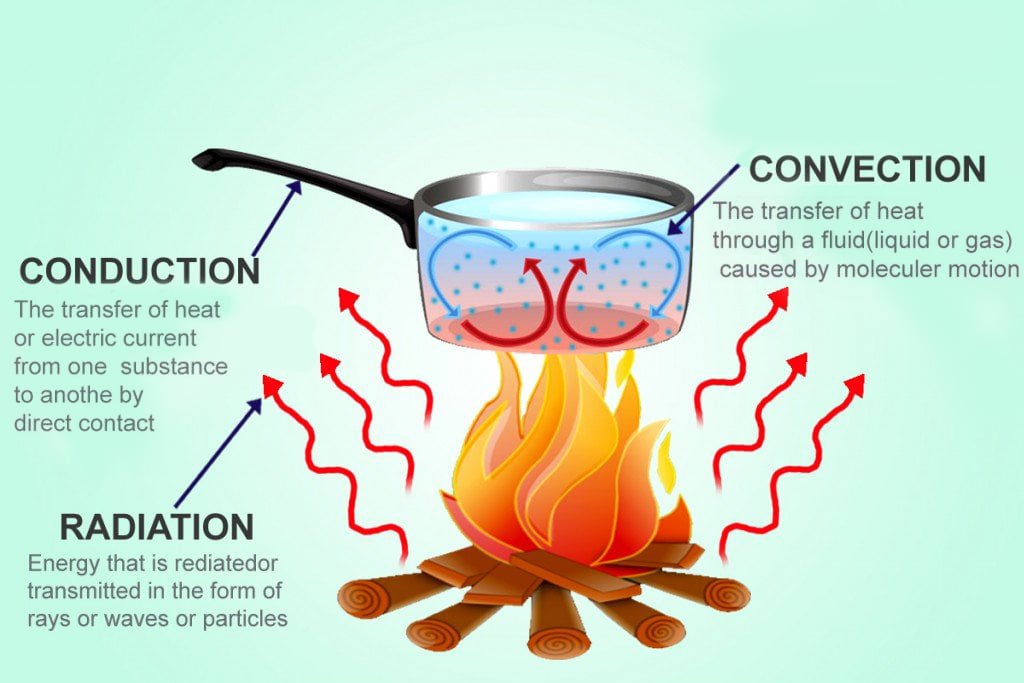
Heat between a source and a recipient can be transferred in three ways: conduction, when the two are in direct contact, like when you touch a hot cup; convection, when the heat is transferred by a medium, usually fluid, like when hot air contacts colder air; and finally, radiation, when the heat is transferred by the means of an electromagnetic wave, which requires no medium to propagate, such as the warmth imparted by the sun’s light that travelled millions of miles through empty space. Microwaves are electromagnetic waves, radiation is how the heat in a microwave oven is transferred.
However, it is still unclear how the heat is distributed throughout the food being subjected to the waves. Microwaves are extremely adept at exciting and vibrating water molecules, and since food is mostly water, the vigorous motion of the molecules creates intermolecular friction, which generates the heat to cook the food item. Although, why the water molecule? Well, because it’s an excellent dipole.
Why Microwaves Target Water Molecules
A water molecule is formed when two hydrogen atoms bond to a single oxygen atom. The molecule, however, is shaped like an upside-down “Y”; the two positive hydrogen atoms are squeezed together on one end, while the highly negative oxygen atom clings to the other. One end of the molecule therefore exhibits a positive charge, while the opposite end exhibits a negative charge: the molecule forms a di-pole.
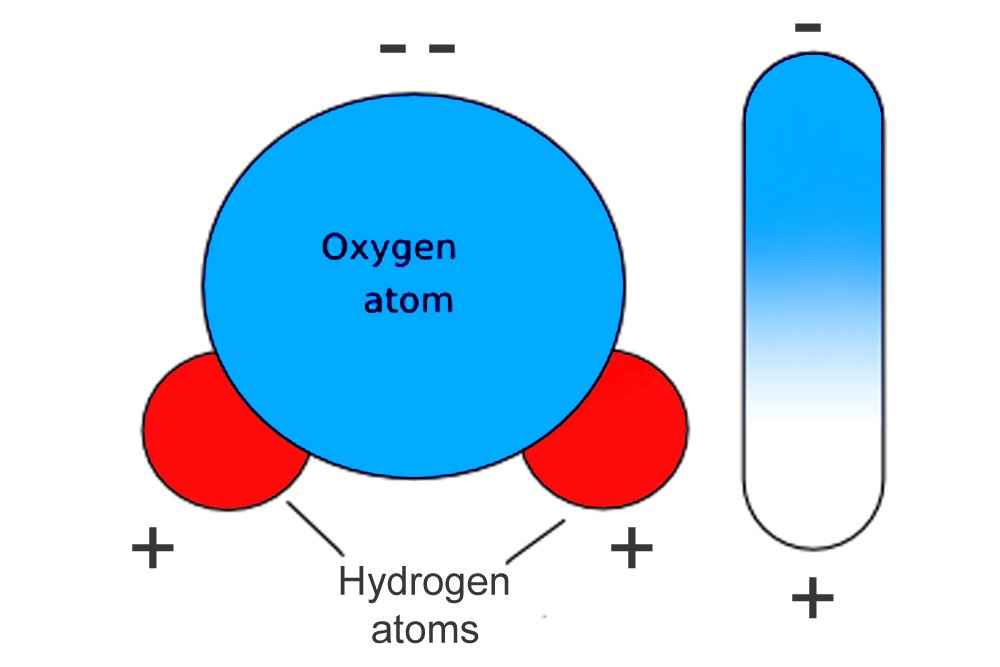
When an electric field contacts a dipole, the dipole tries to align itself with the field’s charge, but microwaves are not just any electric field; they are a rapidly alternating one. As the field alternates, so does the dipole’s alignment, which is to say, the dipole rotates. The water molecule set in such vigorous rotation by the microwaves sets off other molecules in vigorous rotation. Soon, every water molecule is excited and rotating. The vibrational energy dispersed raises the temperature of the food item.
Why Microwaves?
The energy of an electromagnetic wave increases with an increase in its frequency, so why don’t we use infrared or ultraviolet rays to cook food more quickly and efficiently? Infrared or ultraviolet rays aren’t used because they are absorbed by the food item’s surface before they reach the water beneath. Microwaves, however, can penetrate to greater depths, where the water lies. Heating the meat with infrared or ultraviolet might make it ‘look’ hot, but the flesh beneath will remain uncooked. That being said, prolonged infrared heating will eventually heat the meat as well, something that is evident in regular heating, when one cooks on a stove. In fact, many ovens are infrared-based.

Then there are radio waves – electromagnetic waves of even lower frequencies. Why don’t we use them? Wouldn’t they be, inferring from the trend, even more penetrative? Radio waves were actually used to heat food even before microwaves! However, because most of modern communication technology came to be based on radio waves, continuing to use them to heat food would have definitely caused electronic interference.
Lastly, while microwave heating is the quickest and most efficient way to cook food, not every food can be heated with equal ease. Obviously, food items suffused with moisture are the easiest to heat. Food items containing sugar or fat, however, require greater effort. This is because the dipole movement of their molecules is much less than the dipole movement of water molecules.

Also, one must avoid microwaving any food item in a metal container. The fact that metals reflect electromagnetic waves is the working principle of radar. If you feed an oven a metal bowl, most of the microwave energy, as though a metallic ship, will be reflected by it.
Remember that microwave ovens are constructed in a way that allows no waves to escape them, so the reflected energy bounces off the walls and is re-reflected by the metal bowl. What ensues is the greenhouse effect: the energy accumulates and causes the temperature inside the oven to reach dangerous levels, which could eventually cause the oven to explode in your face. If the danger of this happening isn’t apparent to you, perhaps you should just stick to microwave popcorn!