Table of Contents (click to expand)
A water cooler works by using a refrigerant to cool water. The refrigerant is compressed, causing it to become hot. The hot refrigerant is then passed through a condenser, which cools it down. The refrigerant is then squeezed through an expansion valve, which further decreases its temperature. The cold refrigerant is then passed through an evaporator, which transfers heat from the water to the refrigerant.
In our universe, heat can travel in only one direction: from a higher temperature to a lower temperature. Therefore, it seems that water coolers, along with refrigerators and air-conditioners, by freezing fluids (air and water), defy one of the most fundamental laws of nature!
Of course, this is untrue. In fact, it’s impossible, yet these machines are ubiquitous today – indispensable, in fact, to some. What magic is then obscured behind those curved walls of metal and plastic that enables the machine to achieve such a transformation?

Recommended Video for you:
Water Cooler Working Principle
The working principle of a cooler, or for that matter, a refrigerator or air-conditioner, is quite simple: introduce the object whose temperature you wish to decrease to an even colder object, so that when the heat from the hot object is transferred to the colder object, the former is rendered cold. Similarly, water is made colder by acquainting it with an even colder fluid. It surrenders its heat to this colder fluid, thus becoming colder itself. Heat is still traveling in the only direction it can, meaning that no fundamental laws of physics are violated.
However, bear in mind that we desire cold water at room temperature or an even lower temperature, which means that this magic liquid must boil — thereby extracting water’s heat — at room temperature. What’s more, it must also freeze at a meager temperature, since it must be refrozen to be reheated. Otherwise, one would be required to refill the machine with more magic fluid each time all of it is heated, thus rendering it unusable for further use.

The magic fluid that boils and freezes at a very low temperature is called a refrigerant. Consider, for instance, one of the most commonly employed refrigerants, which is called R22. While water boils at 100 degrees Celsius, R22 boils at an astounding -40.8 degrees Celsius! However, refrigerants are not amazing because they boil from a liquid into a vapor or condense from a vapor into a liquid at a low temperature, but rather because they do so very rapidly and seamlessly. How and why is irrelevant to our present discussion. Now, let’s wrap our heads around how the components of a water cooler exploit this property to cool water.
The Process
The water is introduced to the cooler by filling a container called the cooler reservoir. The reservoir is effectively a mini-fridge and is therefore often called the bank fridge. The reservoir is surrounded by coils in which the refrigerant flows.
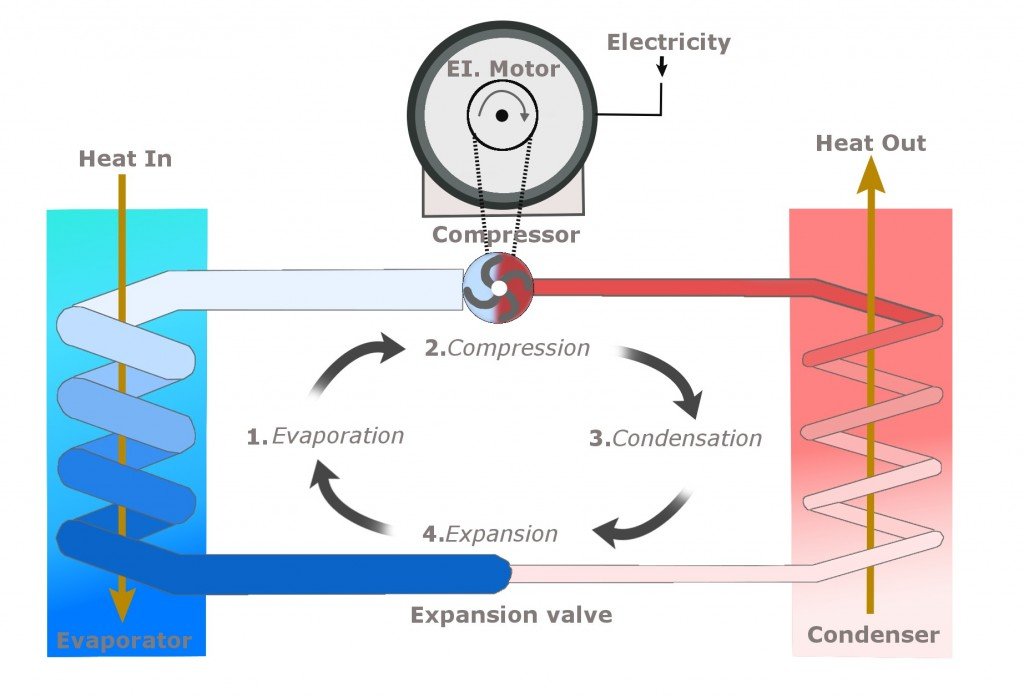
The cooler has four major components: the compressor, the condenser, the expansion valve and the evaporator.
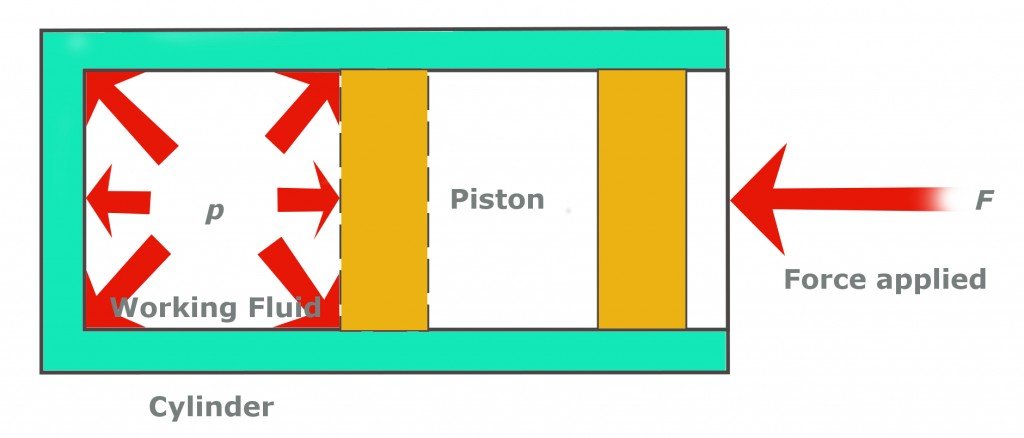
First, the low-pressure and low-temperature refrigerant gas is pumped into the compressor. The compressor, as the name suggests, compresses the gas, thereby raising its pressure. What the compressor does, by pushing the piston over the gas, is reduce the volume that the same number of molecules previously occupied. This causes them to collide more frantically with each other. These collisions elevate the pressure and temperature of the gas. This is exactly why a bike pump becomes hotter when we pump it vigorously.
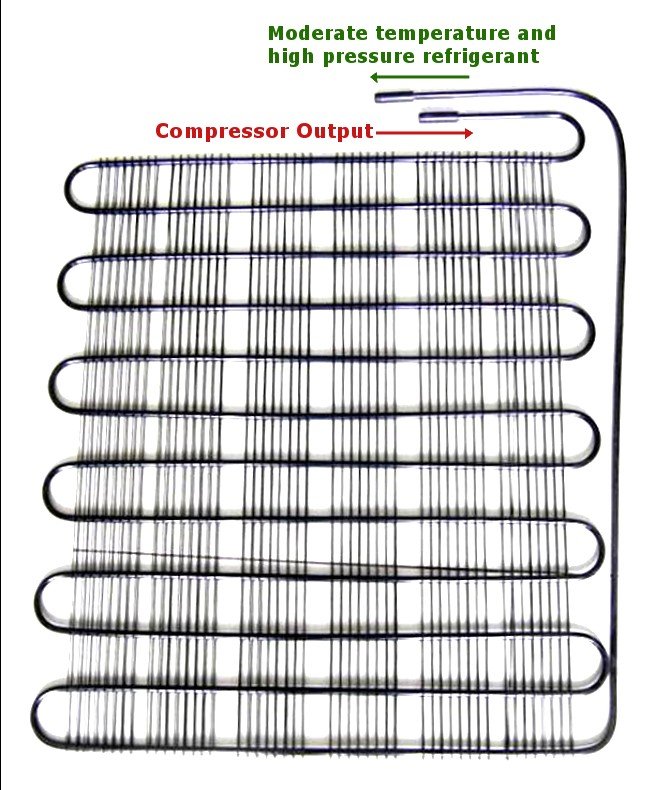
The high-pressure, high-temperature gas is then passed onto the condenser. As the name suggests, it condenses the gas, meaning that it decreases its temperature. This is achieved by making it flow in long, circuitous coils. The area of the tubes is optimized to achieve maximum condensation. An additional loss of heat is achieved by blowing on the pipes with a fan. The condenser causes the gas to lose its heat in the same way that blowing on your hot coffee makes it more tolerable. The extracted heat is vented out into the surroundings, which is why the back of your fridge is always so warm.
The gas is now converted into a liquid of moderate pressure and temperature. The temperature of this refrigerant is further decreased by squeezing it through an expansion valve. An expansion valve is very similar to the nozzle of a spray can. The liquid inside the can is pressurized, but when one pushes the nozzle, the liquid spurts out into a region of low pressure. When the compressed liquid enters such a region, it immediately expands. This expansion, representing a decline in pressure, simultaneously causes a drop in temperature. You have probably experienced how cold the liquid is that leaks from the nozzle incidentally. Therefore, our refrigerant, after exiting the valve, becomes a cold, low/moderate-pressure liquid.
Now, the final component, the component that produces our cold, thirst-quenching water. This last component is called the evaporator. The evaporator in an air-conditioner is a system that comprises a fan to suck in the air of the region intended to be cooled. The temperature of this air is significantly higher than the boiling point of the refrigerant. The cold refrigerant enters the evaporator in pipes, which are exposed to the “warm” air the evaporator sucked in. Heat flows in only one direction. The heat of the air is transferred to the refrigerant, thus making it hotter. The air, now divested of heat, is recirculated into the region through a vent. Voila! Cold air!

The image below neatly summarizes the entire process of cooling.
 However, a water cooler doesn’t necessarily employ a separate, dedicated mechanical unit to evaporate the cold refrigerant. The coils that surround the reservoir often form the condenser itself. The “warm” water surrounded by cold coils transfers its heat to them, and what pours out of the faucet is cold and rejuvenating water. The refrigerant in the pipes, now heated, is pumped into the compressor and the cycle repeats.
However, a water cooler doesn’t necessarily employ a separate, dedicated mechanical unit to evaporate the cold refrigerant. The coils that surround the reservoir often form the condenser itself. The “warm” water surrounded by cold coils transfers its heat to them, and what pours out of the faucet is cold and rejuvenating water. The refrigerant in the pipes, now heated, is pumped into the compressor and the cycle repeats.













