Table of Contents (click to expand)
Carbon dating works by measuring the amount of radioactive carbon in a sample. The amount of radioactive carbon in the sample is used to calculate the age of the sample. The age of the sample is calculated by using the half-life of the radioactive carbon. The half-life of the radioactive carbon is 5,730 years.
Carbon is indispensable to biological life. All life on Earth is based on it. If it weren’t for the amiability of carbon, simple organic matter couldn’t have evolved to achieve the extraordinary, inscrutable complexity it now boasts: the complexity to develop a system to sense, to breathe, to digest, to excrete and in a lean, hairless primate, even a system to think.

However, a tiny percentage of this carbon is radioactive! Measuring the quantity of this radioactive carbon in organic matter allows us to determine its age; the method of doing so is called radioactive carbon dating or, simply, carbon dating. Here’s how it works.
Recommended Video for you:
Carbon-14
Carbon has a twin brother that only a few know about. Our planet is constantly pelted with high-energy cosmic rays hurled by the sun. These rays, which team with neutrons, react with the nitrogen in our atmosphere to produce carbon-14 or C-14 atoms, an isotope of the carbon-12 or C-12 atom.
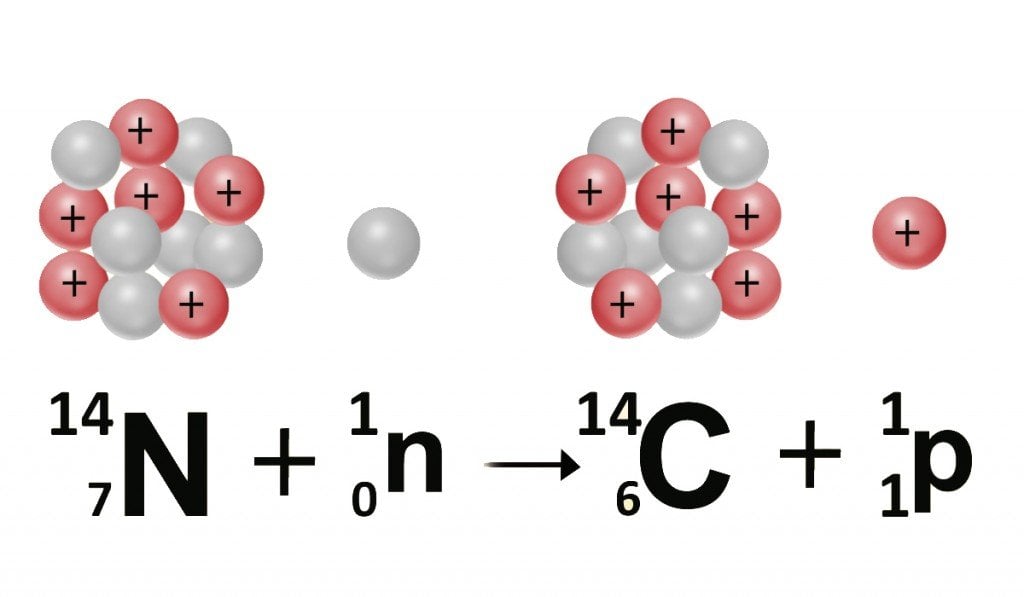
An element and its isotope exhibit the same electric properties, but different physical properties. This is because both elements comprise the same number of protons and electrons, but a different number of neutrons. The twins are then identified by different denotations, highlighting the number of neutrons, which is appended to the element’s symbol. C-12 has 6 neutrons, while C-14 has 8 neutrons; both, however, have 6 protons and electrons.
The key things about C-14 are that it is radioactive, that it is unstable, which forces it to emit particles and therefore decay over time.

The Principle Of Carbon-Dating
The radioactive carbon will react with oxygen in the atmosphere to produce radioactive carbon dioxide. This radioactive carbon dioxide is breathed in and stored by plants, which are consumed by herbivores, who are preyed on by carnivores or omnivores, such as humans. The carbon content of every organism under the atmosphere therefore is composed of mostly C-12 atoms and a minuscule number of C-14 atoms.
The organisms, while they do consume carbon, also expel it when they exhale. The transaction or the cycle of producing, consuming and expelling C-14 atoms occurs in a way that, even though the amounts of C-12 and C-14 atoms in the environment and in an organism may vary, their ratio will remain the same. This is the working principle of carbon dating: despite the transactions, a living organism maintains the same ratio of C-14 to C-12 atoms as found in the environment.
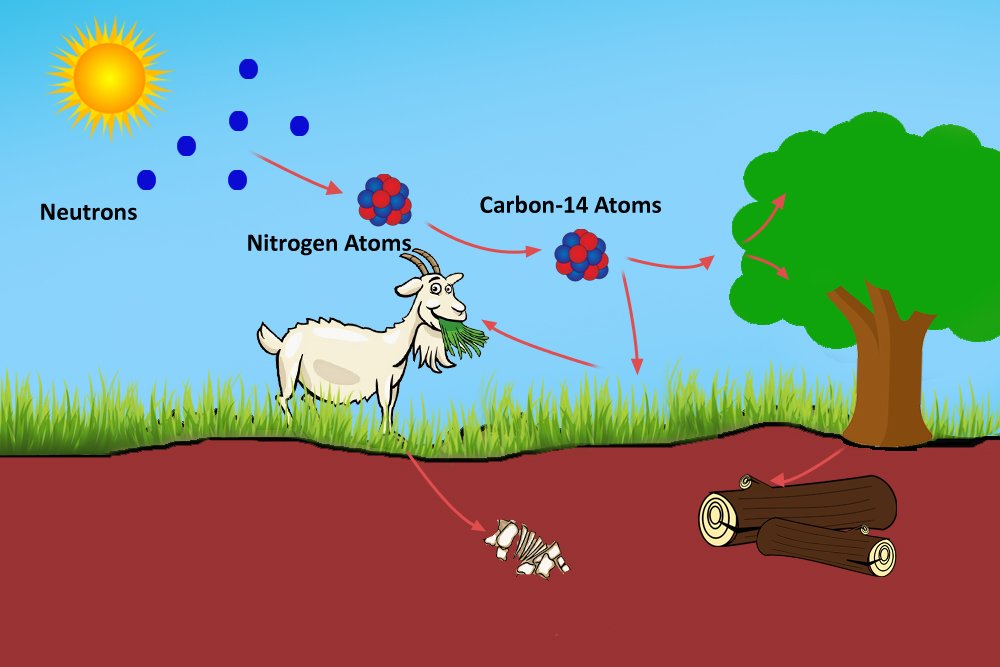
However, when an organism dies, it ceases to consume carbon. Now, because C-14 is radioactive, it begins to decay. The ratio of C-14 to C-12 atoms in the organism now decreases. The older the organism, the more C-14 is decayed, so the smaller the ratio. This ratio is used by archaeologists to date, say, a tree or a fossil.
They refer to the following equation to measure a sample’s age:

The equation dictates the decay of a radioactive isotope. Here, Nᵒ represents the number of atoms of the isotope in the sample at t=0 or when the organism, a part of whom now forms the sample, died, while N represents the number of atoms left after time t has passed.
Remember that the ratio of C-14 to C-12 atoms in the organism and the environment is the same when it is alive. The knowledge of this ratio, which we already possess, allows us to obtain the value of Nᵒ, the original number of C-14 atoms. The current value N, however, must be measured. The C-14 atoms in the sample are counted by delicate instruments, such as beta-counters and mass accelerator spectrometers.
λ is an element constant whose value for C-14 is 8,267. The time t that has since passed or the age of the sample can be obtained by rearranging the equation:

Is Carbon Dating Reliable?
The radioactivity of an element is measured in terms of its half-life: the time it takes to decay half of its constituents. The half-life of C-14 is 5,730 years, which means that it becomes half of what it originally was in 5,730 years, one-fourth in 11,460 years, one-eighth in 17,190 years and so on.
Extend the trend and one discerns that accurately measuring that the entirety of the atoms decays or, at least the percentage below which they become undetectable, after around 50,000 years. Consequently, dating a sample older than 50,000 years may produce erroneous results.
Composite techniques have been devised that combine carbon dating with techniques to calibrate and extend its scope, but even those techniques are inherently fallible. Carbon dating is therefore only unquestionably accurate for a few thousand years; any results beyond that frame is questionable. This is the major limitation of carbon dating.

What’s more, carbon dating seems to be based on a fallacy. It is fundamentally based on the assumption that the ratio of C-14 to C-12 atoms in the environment has always been the same throughout each and every Age.
This is certainly not true. Since the Industrial Revolution, in particular, we have diluted the amount of C-12 atoms in the environment by shamelessly dumping into it an alarming quantity of carbon dioxide, produced by the burning of fossil fuels. An increase in C-12 means that the ratio is now reduced, which means that the age of a sample will measure to be older than it really is!
Conversely, nuclear explosions produce tremendous amounts of C-14, so the plethora of nuclear tests we have conducted has increased its amount in the atmosphere. This increases the ratio, causing the age of a sample to measure younger than it really is.

Still, with the knowledge of the amount of deviation that an increase or decrease in C-14 atoms will cause, we can account for these discrepancies by simply subtracting or adding the error from or to the apparent age to obtain the real age. Again, carbon dating might not be unquestionably accurate, but it’s good enough.