Plants might utilize quantum mechanics to prevent decoherence during photosynthesis, which makes the process more efficient.
In 2007, a research team composed of researchers from the University of Chicago and the University of Washington in the USA, as well as the Institute of Physics of Charles University in Prague, proposed that plants utilize quantum mechanics and perform quantum computation. The researchers proposed that quantum mechanics, which many still consider the domain of sci-fi (for the moment at least), was taking place in your garden.
This was initially considered so preposterous that when the paper claiming quantum capabilities for plants was published, scientists laughed. Quantum machines, such as quantum computers, required specialized conditions like sub-zero temperatures and a pristine vacuum to work. Given those intense conditions, it seemed impossible that plants living in the real world, where temperatures can reach 40 degrees Centigrade and molecules like water and carbon dioxide are constantly hitting everything, might also be able to engage in quantum mechanics.
However, as scientists investigated this ridiculous claim further, the evidence for the quantum nature of plants only became stronger.
So, what do plants use quantum mechanics for and how do they do it?
Recommended Video for you:
Where Is Quantum Mechanics Used In Photosynthesis?
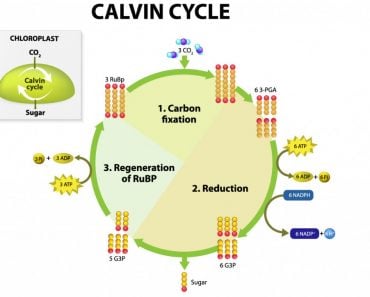
Photosynthesis is one of the backbones of life on our planet. It supplies the world with oxygen and food using carbon dioxide from the air. All of this is powered by the energy of the sun.
Molecules called chromophores (one of which is chlorophyll) capture photons present in sunlight.
Note: I will write “plants” when I want to refer to photosynthetic organisms, but remember that plants aren’t the only ones that perform photosynthesis. Algae, protists and some bacteria do it too!
When the photons hit the chromophores, they pass their energy onto an electron of the chlorophyll molecule. This electron is now energized, like an adult who has just had their morning coffee. The energy combined with the electron is now called an exciton.
This exciton must make its way to a place called the reaction center. The reaction center is where the exciton will deposit its energy, which will eventually go on to become ATP, the form of energy that plants can use to make food (and do other stuff). Many other processes must happen before ATP is formed, but that goes beyond the scope of this article.
Getting the exciton to the reaction center isn’t a straightforward process, which is where quantum mechanics comes into the picture.
Photosynthesis Can Avoid Decoherence
The classical view is that the exciton might jump from one chromophore to another until it finally finds its way to the reaction center. This is popularly described as a “drunken walk”, a random “hopping” around until reaching a desired destination.

However, contrary to that view, scientists noticed that the exciton is never actually lost. It never veered off and deposited the energy elsewhere, and almost always found its way to the reaction center. If it is a “drunken stumble”, then the exciton should probably get lost once in a while. This “getting lost” does happen in biological systems, so why not here?
The 2007 paper proposed that photosynthetic organisms were able to prevent something called decoherence.
What Is Superposition And Decoherence?
In classical physics, if you’re standing in line at the coffee shop, you can’t simultaneously be at work or in your car driving. You can only be in one place at one time.
This is not the case for quantum particles. If you were a quantum particle, there would be different probabilities for you being at either at the coffee shop, work or in your car.
When scientists want to know where you—the quantum particle—can be found, they have to measure. When they do that, they force you to be in one place. However, until they exactly measure where you are, you are simultaneously at the coffee shop, in your car, and at work.
The act of being in multiple places at once is called superposition.

This is something that photosynthetic processes might also use in getting the exciton to the reaction center.
Since the exciton, a quantum particle, can be in multiple places at the same time, it can take every route to the reaction center simultaneously. Imagine this process as if you were caught in a maze. It would take you less time to get out of the maze if you could split yourself into many different versions of yourself and explore every route simultaneously, rather than exploring every possible route one at a time, and consequently getting lost.
The exciton is in a maze and it needs to reach the reaction center, so its quantum properties come to the rescue and allow it to be everywhere at once, and thus find the fastest route to the reaction center.
But remember how I said that measuring a quantum particle would force it to be in only one place? That measurement actually consists of a photon hitting the quantum particle (which is why vacuum is so important to make quantum things happen in labs). In fancy quantum terms, the photon makes the “wave function collapse” and the quantum particle begins to act like a boring classical particle, located in one place at one time.
This change from quantum to classical is called decoherence. The opposite is coherence, when the quantum particle is still acting both as a wave and a particle and doing very weird quantum things.
Photosynthetic Organisms Seem To Avoid Decoherence
Now, life is messy and hot and filled with way too many molecules bouncing around. One would reasonably expect that all these bouncing molecules, not to mention the photons of sunlight raining down, would drive the exciton into decoherence.
However, if the 2007 paper and subsequent work in the field is to be believed, then yes, photosynthetic algae and bacteria do manage to avoid decoherence. Precisely how they do this remains the question. This question regarding the mechanism has made many doubt whether life can use quantum mechanics at all!
Are The Quantum Mechanics Of Photosynthesis Exaggerated?

However, as with any new and bold claim comes skepticism and controversy. The main dispute is over how to interpret the evidence from experimentation. The 2007 paper interpreted certain “beats” that were found through their experimentation as evidence for quantum coherence. In 2013, researchers at the University of Colorado, Boulder contested that those “beats” weren’t actually evidence of quantum activity. Instead, they were nothing but vibrational energies of the chromatophores. Every molecule has a certain amount of energy, making it vibrate with its own unique frequency. Classical versus quantum interpretation is at the heart of this debate.
Even so, biological processes are rarely so black and white. There is a possibility that, while vibrational energies are dominant, part of the mechanism might also be a part of the quantum realm.
In the end, understanding exactly how plants transfer their energy might help us engineer new cutting-edge technologies, such as solar power, to be even more efficient.
References (click to expand)
- Engel, G. S., Calhoun, T. R., Read, E. L., Ahn, T.-K., Mančal, T., Cheng, Y.-C., … Fleming, G. R. (2007, April). Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature. Springer Science and Business Media LLC.
- Tiwari, V., Peters, W. K., & Jonas, D. M. (2012, December 24). Electronic resonance with anticorrelated pigment vibrations drives photosynthetic energy transfer outside the adiabatic framework. Proceedings of the National Academy of Sciences. Proceedings of the National Academy of Sciences.
- Is photosynthesis quantum-ish? - Physics World. Physics World