Table of Contents (click to expand)
The third law of thermodynamic states that as the temperature of a system approaches absolute zero, its entropy becomes constant, or the change in entropy is zero. The third law of thermodynamics predicts the properties of a system and the behavior of entropy in a unique environment known as absolute temperature.
The entropy of a bounded or isolated system becomes constant as its temperature approaches absolute temperature (absolute zero).
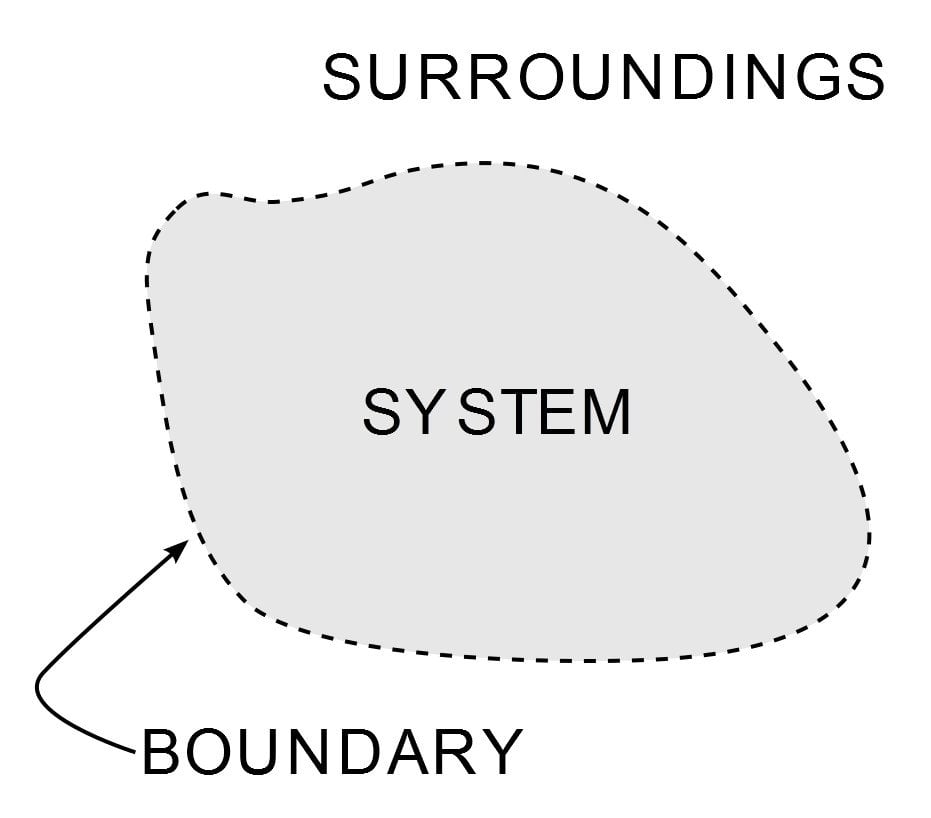
Thermodynamics is one of the most important and widely studied branches of physical science. Other than tormenting mechanical engineering students for most of their academic lives, its ubiquity is seen from the cold breeze of my air conditioner to one of the pinnacles of the industrial age – the steam engine. Its implementation is governed by three laws, which are known as the Laws of Thermodynamics. The laws define how work, heat and energy affect a system. A system is any region in the Universe that is finitely bounded across which energy is transferred. Everything outside this boundary is its surroundings.
Recommended Video for you:
What Is Entropy?
While the first law of thermodynamics implies that the Universe began with finite usable energy, where a system drawing energy will partly spend it in doing work and partly spend it through increasing its internal temperature, the second law explores its implications. This includes the conversion of this finite usable energy into unusable energy; for instance, the formation of matter occurring billions of years ago due to the condensation of energy that the Universe started out with. In this process, the finite usable energy is now converted to unusable energy.
This unusable energy is measured by something called “Entropy”, a barometer for measuring randomness or disorder in a system.
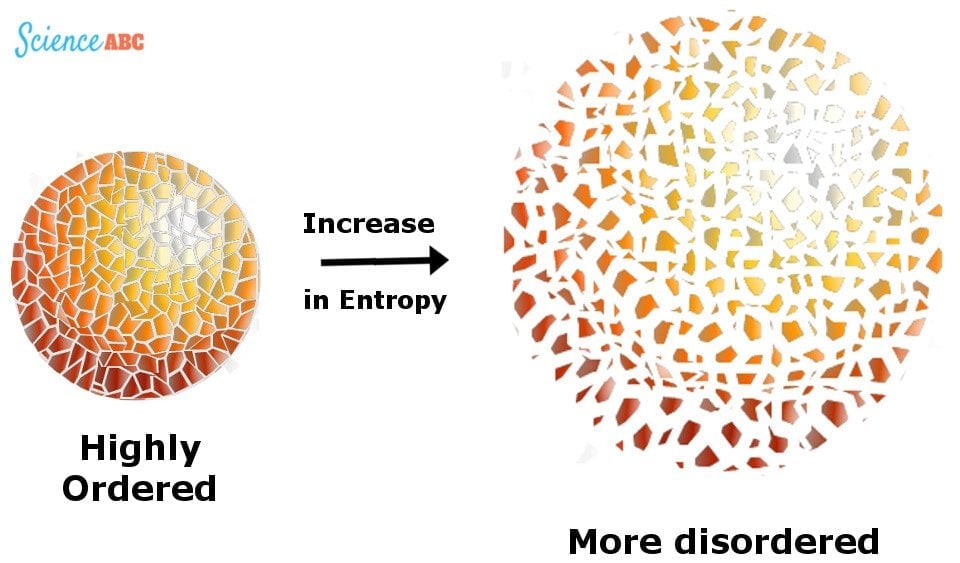
The Universe is like a room filled with clothes that are lying around in an unorganized way. The entropy of this system increases as more and more clothes are used and discarded, supplementing the mess, unless the inhabitant makes an effort to pick them up and organize them, which reduces this disorder.
Considering the Universe as one system, there is nothing in its surroundings to derive energy from, so with all its energy converted to unusable energy, all that is left behind is a cold, dark place. This is called the heat death and is one of the ways the Universe could end. A bounded system like our Universe possesses finite sources of energy, such as its bright stars, which will burn for aeons before surrendering to the cruel laws of nature.

What’s The Third Law Of Thermodynamics?
The third law of thermodynamics predicts the properties of a system and the behavior of entropy in a unique environment known as absolute temperature. The absolute temperature is the lowest temperature known and sets a lower limit to the Universe’s temperature range.
How cool is that! No, seriously, how cold is it? The absolute temperature is 0 Kelvin, the standard unit of temperature or -273.15 degrees Celsius! Absolute temperature is also known as absolute zero in some circles and countries. This scale will give you an idea.
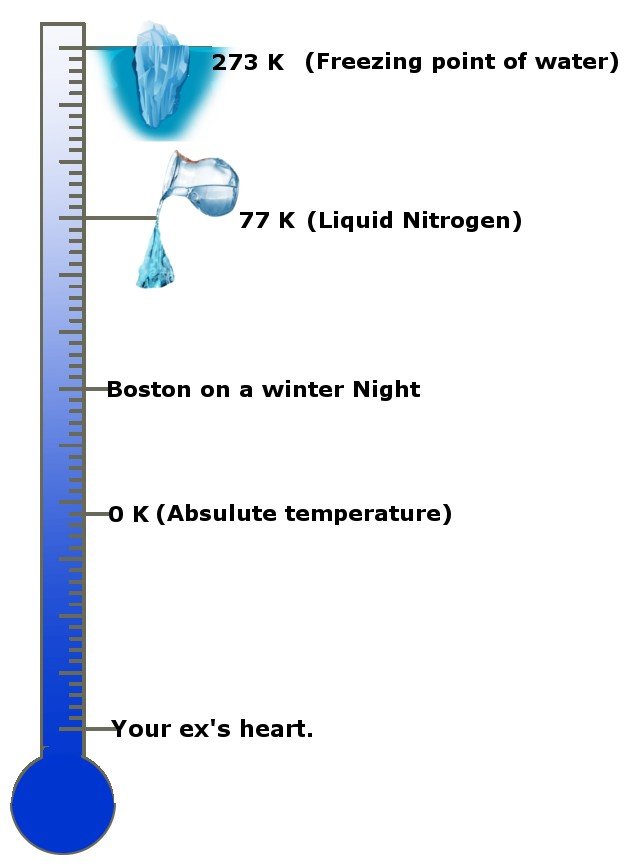
The third law states that as the temperature of a system approaches absolute zero, its entropy becomes constant, or the change in entropy is zero.

The statement is represented by this equation, where T resembles the temperature and delta S is the change in the system’s entropy. The denotation “tends to zero” represented by an arrow pointing towards zero implies that as the temperature decreases to an infinitesimal value, the system achieves constant entropy by drawing energy from its surroundings, but as the first law dictates, part of this energy will add to the system’s internal energy, thereby denying a constant entropy state.
Importance Of The Third Law Of Thermodynamics
The third law is rarely applicable to our day-to-day lives and governs the dynamics of objects at the lowest known temperatures. It defines what is called a ‘perfect crystal’, whose atoms are glued in their positions. The perfect crystal thus possesses absolutely no entropy, which is only achievable at the absolute temperature.
The concept of entropy has also been popular in some theories defining the continuous flow of time objectively, such as the linear increase in the entropy of the Universe.
Ideally, at 0 Kelvin, the entropy changes for reactions regarding the formation of matter will be zero, although practically all matter manifests some amount of entropy, owing to the presence of the tiniest amount of heat. The coldest we have measured is 3 K, in the distant depths of the Universe, beyond stars and galaxies.
In other words, enjoy the summer while it lasts!
Read More: