Table of Contents (click to expand)
The photons produced by a source of light come from the energy created when an electron transitions from a higher energy level to a lower energy level.
If you peep inside a lamp or a tube light, you wouldn’t find a hidden army of uncountable photons ready to ambush on the switch’s command. In the case of a bulb, the most you’d find is a metal filament. Whereas, in the case of a tube light, you’d find, well…nothing. Where then do the photons come from? Do they spring from thin air? Actually, yes. The photons do pop into existence from absolutely nothing. Here’s how.

Recommended Video for you:
More Energy=More Light
Human sight has relied on the blessing that is light since time immemorial, although light ceased to be a “blessing” since we domesticated fire and invented bulbs. However, while we usurped the role of God, people have rarely understood how the light in the bulb was being produced. For instance, a bulb produces light as the current supplied to it – through resistive heating – heats the tungsten filament hanging in the middle. The tungsten heated to a temperature of 2500 degrees Celsius produces an ocean of photons that flood your room. However, why should heated objects emit light?
Also, if heated objects do emit light, why are humans and other warm-blooded animals so difficult to find in a completely dark room? Reasoning that they’re not warm enough has some truth to it; warm-blooded creatures do emit light, but due to their paltry heat, it isn’t much. As a result, the light is not “visible”. However, it is embarrassingly lazy to merely blame it on the magnitude of energy. This reasoning is incomplete. LED bulbs or CFL tubes aren’t nearly as hot as incandescent bulbs, and yet they are equally bright.

Even though we have bent light to our whims for thousands of years, it was only last century that we gained a comprehensive understanding of how photons are created.
Photon Definition
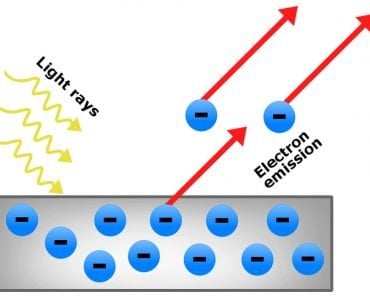
Light is an electromagnetic wave. A photon is described as an excitation of the electromagnetic field. To excite the electric field, which then excites the magnetic field (according to Maxwell’s laws), which re-excites the electric field and so on, we must first excite, as the field’s name suggests, electrons!
Electrons reside inside atoms in different energy levels. When we excite electrons by, for example, in the case of a bulb, heating the tungsten atoms, we elevate them to higher energy levels. However, nature seeks stability; electrons abhor climbing to higher levels. To achieve stability, electrons descend back to their original or even lower levels. When an electron makes this downward leap, the atom emits a photon. As millions and billions of electrons simultaneously descend to lower levels, the tungsten unleashes a massive torrent of photons.
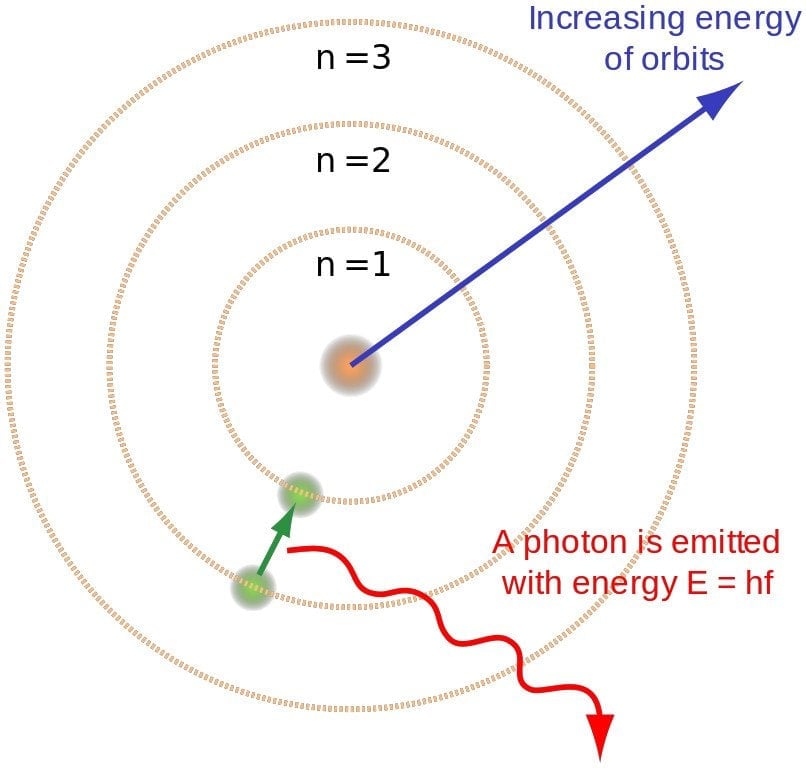
Energy Of A Photon
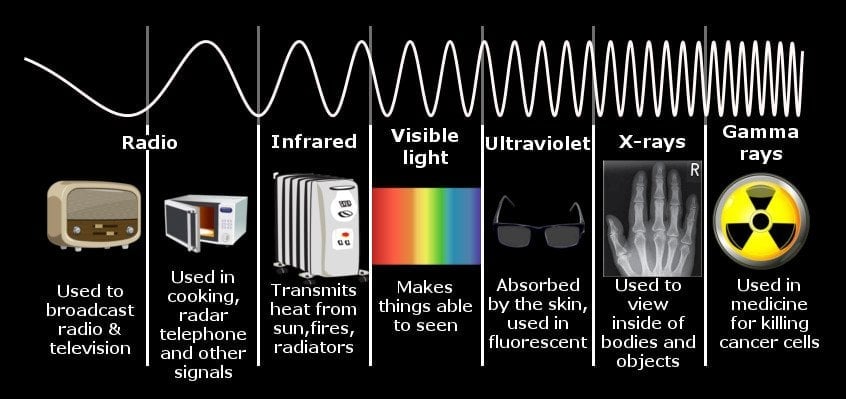
Whether the light produced will be visible or invisible depends on the electromagnetic wave’s frequency or the energy of the photon. The electromagnetic spectrum can be divided into seven categories. The human eye can only detect one of them, so it is rightly called the visible spectrum. The frequency of an electromagnetic wave or a photon’s energy is directly proportional to the distance jumped by an electron.
When electrons, such as the electrons of a tungsten filament, make a downward leap that translates to a frequency lying within this spectrum, the photons produced are visible. Leaps to a space outside this range, whether larger or smaller, will produce photons that are invisible. This is why the light that warm bodies produce cannot be detected by the human eye, but it can be detected by devices attuned to low-frequency infrared waves. The leaps the electrons that produce these photons make aren’t large enough. So is the case with the radio waves transmitted and received by our phones. Beyond the visible spectrum lie waves characterized by longer leaps, such as ultraviolet or X-rays, waves emitted by stars and other exceedingly high-energy phenomena, such as quasars and supernovae.
It makes no difference whether one excites the electrons by subjecting them to heat or electricity; what matters is the magnitude of their elevation. This realization led us to abandon our notion that more heat equals more light. While it is true, as exemplified by stars, it is inefficient. For this reason alone, incandescent bulbs – which waste a tremendous amount of heat energy – are tragically inefficient when compared to CFLs, which excite and elevate electrons by simply passing electricity through tubes that contain vapors of argon and mercury.
So, light or a photon is produced when an electron transitions from a higher energy level to a lower energy level. However, if you were to peek inside an atom, would you find a hidden army of uncountable photons, ready to ambush on the electron’s command?
Well, no. As explained, an electron jumps to a lower energy level to achieve stability, that is, it loses the energy that forced it to climb in the first place. The universe, unlike in the case of heat energy, cannot squander this organized energy; it must put the extra energy to use some. The result is the instantaneous creation of a photon; it literally pops into existence out of sheer nothing, whatever that is.
Humans are morbidly curious: one might wish to dig deeper, but this is the deepest one can dig. To ask a deeper “why” is tantamount to asking, “why are smaller objects attracted to massive objects?” or “why is the speed of light approximately 3,00,000 km/s?” That is how gravity fundamentally works and that is the velocity with which an electromagnetic wave travels in a vacuum. Photons are created when electrons make a downward transition because that is how quantum electrodynamics works, at least in this universe. At a level so fundamental, physicists, as Cornell physicist David Mermin commanded (although the maxim is misattributed to Richard Feynman and sometimes Paul Dirac), “shut up and calculate”.