This physical law applies everywhere, whether it’s a mountain range on the equator or in polar regions – regardless of its geographical location on the planet. So, if you had any plans to ride in a soaring aircraft until you experience the soothing warmth of the sun, you should just forget that idea entirely, or else get ready to leave the planet and experience the not so pleasant consequences of interplanetary space!
If you think about it, if you really step back and consider it logically, doesn’t it seem counter-intuitive that the higher up you go, the colder it gets? Shouldn’t it be the exact opposite? After all, by going towards high-altitude regions, you’re essentially moving closer to the Sun, i.e., reducing the distance between yourself and the Sun, even if it’s an incredibly minuscule amount. Going by this reasoning, one should feel hotter on hills and mountains, but as we all know, that doesn’t happen. Why is that?
In short, it’s due to atmospheric pressure.
Recommended Video for you:
A Little Something About Atmospheric Pressure
To start with, you need to understand that the Sun is incredibly far from the Earth; so moving to higher altitudes makes no difference whatsoever in terms of the heat it provides. You would have to leave the planet altogether and continue moving much closer to our star to experience a noticeable change in the heat your skin experiences. Therefore, moving to mountainous regions doesn’t bring you a significant distance closer to the Sun, at least in terms of heat.

Now, let’s talk about the atmosphere. As you probably already know, it’s composed of a mixture of gases that envelops the Earth. Since the atmosphere envelops the Earth, i.e., it can be found practically everywhere on the planet, it also exerts a certain amount of pressure, which is known as the atmospheric pressure (sometimes also referred to as ‘barometric pressure’). To put it simply, just think of the atmosphere as a load that’s pushing down on the planet; the pressure with which it pushes down is what scientists call atmospheric pressure.
Now, let me tell you, this is no ordinary load; it’s a huge mixture of gases and dust particles that weighs a huge amount. It’s akin to holding a small car on top of your head at all times. In theory, humans, and almost everything on the planet should be crushed by the sheer weight of the atmosphere.
However, it’s quite interesting to note that the value of atmospheric pressure is not same everywhere – it changes with altitude. This is why a number of things happen – or don’t happen – at higher elevations.
How Atmospheric Pressure Changes With Altitude
As mentioned earlier, the atmosphere is simply a mixture of gases that hover above the Earth’s surface. At sea level, the value of the atmospheric pressure is 14.7 pounds per square inch (Source), but as you go up into higher altitude regions, it begins to decrease and becomes almost non-existent beyond a particular point.
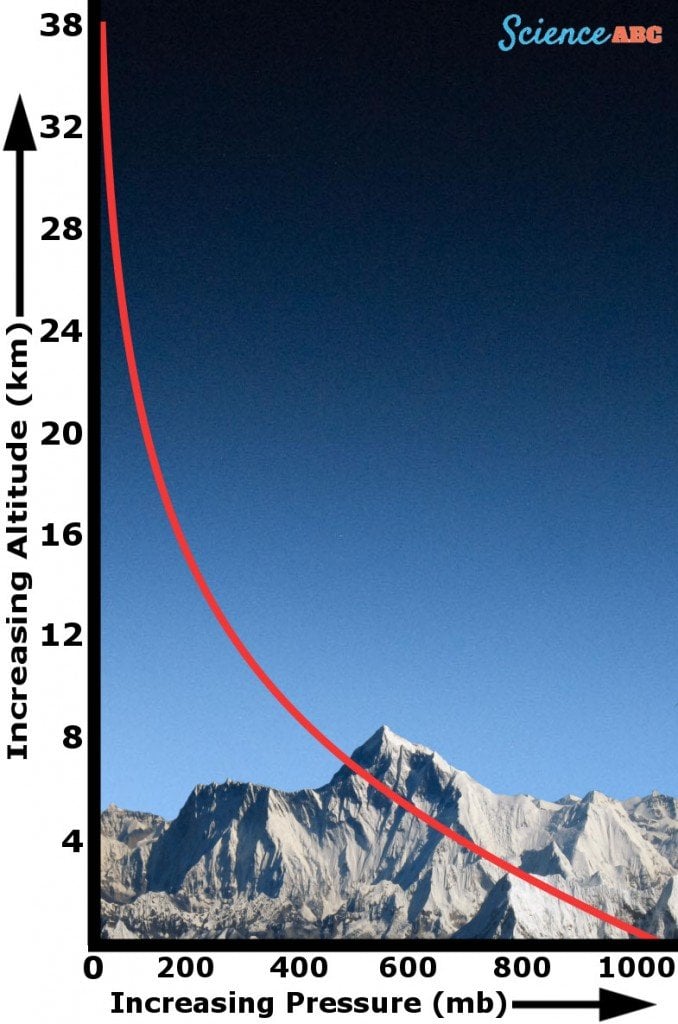
The atmospheric pressure has this trend due to the decreasing quantity of air molecules as the altitude increases. Consider it this way: the higher you go up, there are fewer and fewer molecules pushing down from above. This is why high-altitude regions experience a much lower atmospheric pressure than regions at sea level.
What Does Atmospheric Pressure Have To Do With Temperature?

Since the atmospheric pressure decreases with altitude, the number of air molecules pressing on the other molecules (at lower altitudes) from above also drops. This leads to an expansion of sorts, in the sense that the molecules below have more room to wander about. This makes them less likely to collide and bounce off neighboring molecules, which results in kinetic energy being distributed over a large area. This brings down the average temperature of the system, i.e., the atmosphere at that altitude.
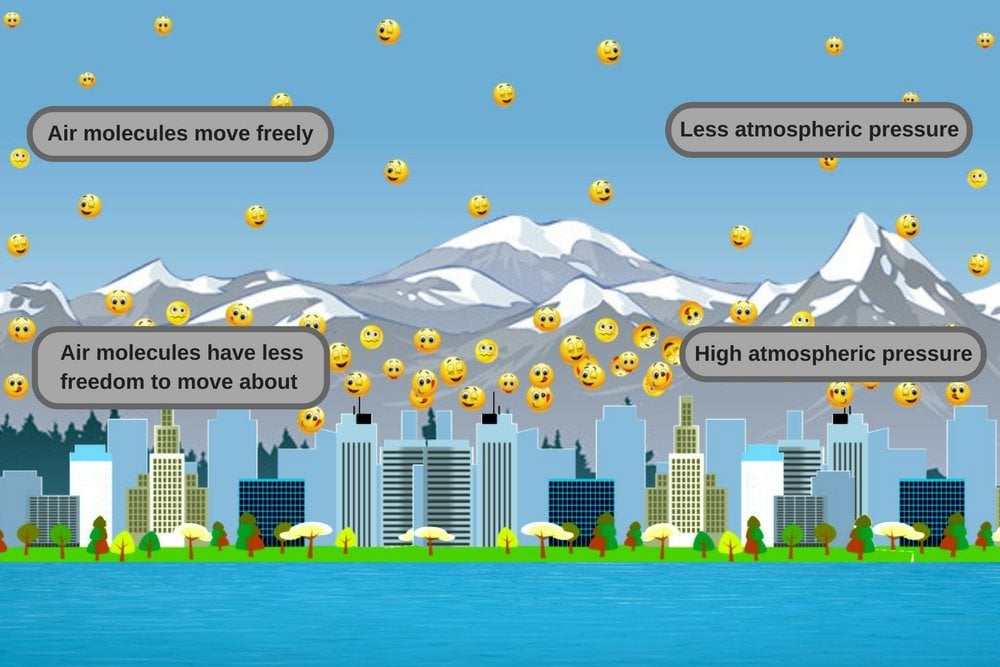
In contrast, in low-altitude regions, air pressure is high, so air molecules don’t have as much freedom to move about. Carrying a lot of energy, they collide with each other more frequently, which causes the temperature of the system to increase. This is why low-altitude areas are hotter than mountainous regions. This physical law applies everywhere, whether it’s a mountain range on the equator or in polar regions – regardless of its geographical location on the planet.
So, if you had any plans to ride in a soaring aircraft until you experience the soothing warmth of the sun, you should just forget that idea entirely, or else get ready to leave the planet and experience the not so pleasant consequences of interplanetary space!