Table of Contents (click to expand)
The simple reason is that the chemical and atmospheric conditions of this region are very good at increasing the effectiveness of ozone destruction by reactive halogen gases.
The simple reason is that the chemical and atmospheric conditions of this region are very good at increasing the effectiveness of ozone destruction by reactive halogen gases.
I remember back in high school, when my science teacher asked if I knew about the ozone hole. She asked if I knew how big the hole was, and where exactly did it exist.
I didn’t know the answers to those questions, but I certainly knew a thing or two about the ozone layer, and how it protected us from the ‘bad’ sunlight.
I now know that the ozone hole is situated roughly above the continent of Antarctica, as the depletion of ozone is the worst there, but do you know why that’s the case?
Recommended Video for you:
A Little Something About Ozone…
Ozone is a molecule containing three oxygen atoms. Molecular oxygen gas, i.e., the one we breathe, contains two oxygen atoms, but ozone contains another. It’s actually a pale blue gas with a pungent smell. It’s highly unstable, and thus very reactive.
Ozone is formed in the upper atmosphere when UV rays from the sun split diatomic oxygen (which is abundant in the atmosphere). The lone oxygen atoms then collide and react with other O2 molecules to make ozone.
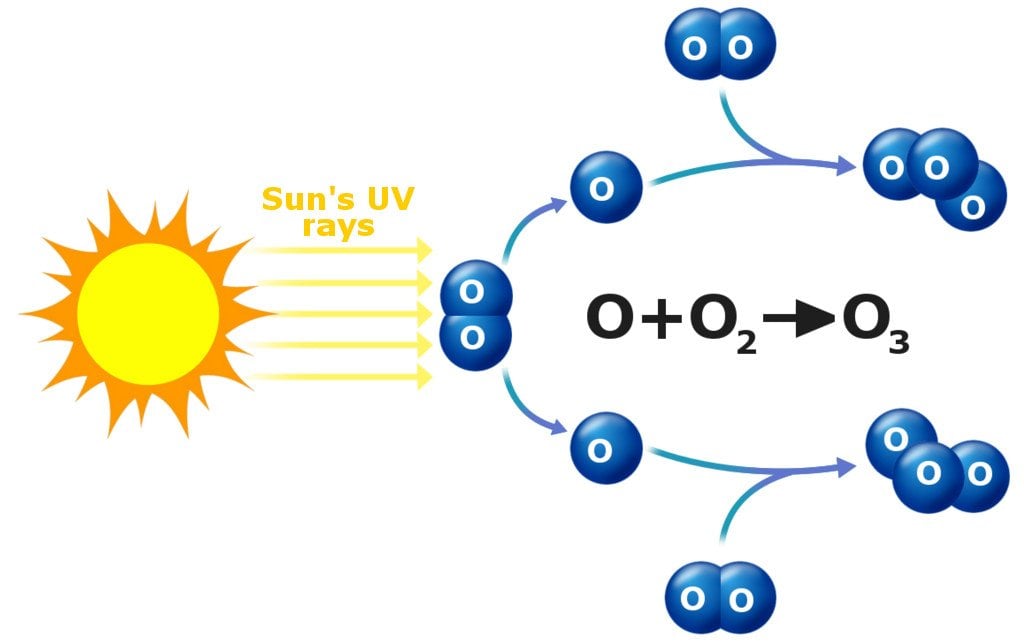
The upper layers of the atmosphere have higher levels of ozone, which is a good thing, because the ozone layer shields the planet from harmful UV rays from the sun.
What Is The Ozone Hole?
The ozone layer has depleted significantly due to certain industrial activities in the past century or so. Ozone depletion actually refers to two related events: a steady decrease of around 4% of the total amount of ozone in the atmosphere, and the severe depletion of stratospheric ozone in late winter and early spring in the Antarctic. The latter is also called the ‘ozone hole’.

Well, first of all, let me tell you that the ozone hole is not really a ‘hole’; it’s just a region of exceptionally depleted ozone in the stratosphere over the Antarctic that occurs at the beginning of the southern hemisphere spring (around the months of August-October).
Why Ozone Layer Depletion Is Prominent Over Antarctica?
The simple reason is that the chemical and atmospheric conditions of this region are very good at increasing the effectiveness of ozone destruction by reactive halogen gases.
In other words, the conditions over Antarctica are the most suited for depletion of the ozone layer.
Firstly, strong winds blowing around the continent form a polar vortex, which isolates the air from the rest of the world from Antarctica. You see, strong winds in the stratosphere form a sort of ring of moving air above the continent, which prevents substantial air motion into or out of the polar stratosphere.
Then, there is the formation of Polar Stratospheric Clouds (PSCs).
Also known as nacreous clouds, these are the clouds that form in the winter polar stratosphere. These are best observed during winters in more northerly latitudes.

These clouds also contribute to ozone depletion over Antarctica, as reactions on the surfaces of solid and liquid PSCs can significantly increase the abundance of the most reactive chlorine gases. These reactions then lead to the formation of certain compounds that chemically destroy ozone in the presence of sunlight.
Antarctica is one of the coldest places in the world, and severe ozone depletion requires just that. In order for ozone depletion, low temperatures must be present over a range of stratospheric altitudes, over large regions and for an extended period of time. Antarctica fulfills all these conditions, which is why ozone depletion is at its worst there.