Sulfates such as SLS and SLES are thought to be harmful to one’s body and skin. Looking at the research can help us clarify the line between marketing myth and scientific fact.
Over the years, sulfates have developed a bad reputation among consumers. They are accused of causing hair loss, skin irritation and being a carcinogen. The sulfate-free shampoo industry claims to be the alternative and healthier choice.
However, is this all a marketing gimmick? And if it is unsafe, then why are sulfates a significant part of shampoo? Is there any evidence of their toxicity?

Recommended Video for you:
What Are Sulfates?
In 1930, Procter and Gamble made the first sulfate-based shampoo and ever since, the ingredient has been integral to shampoos. Sulfates are a broader term used for synthetic sulfate-based chemicals, in our case, sodium lauryl sulfate (SLS) and Sodium Laureth Sulfate (SLES).
Sulfates are surface-active agents classified under anionic surfactants. They are good cleansing and foaming agents, which is why shampoos, soaps, toothpastes and other cleaning products contain sulfate.
SLS And SLES: What Are They?
Sodium Lauryl Sulfate (SLS) is an alkyl sulfate. It acts as a surfactant in cleaning products. Surfactants are compounds that reduce the surface tension between two phases, in this case, between water and air. The phase can be between liquid-liquid, liquid-gaseous or liquid-solid phase.
Surfactants also have foaming, dispersing and detergent properties.
SLS is a harsh cleansing and foaming agent, which increases the chances of skin irritation when applied to the skin for a longer duration. Due to this, Sodium Laureth Sulfate (SLES) is often used in shampoos, as they are less of an irritant than SLS.
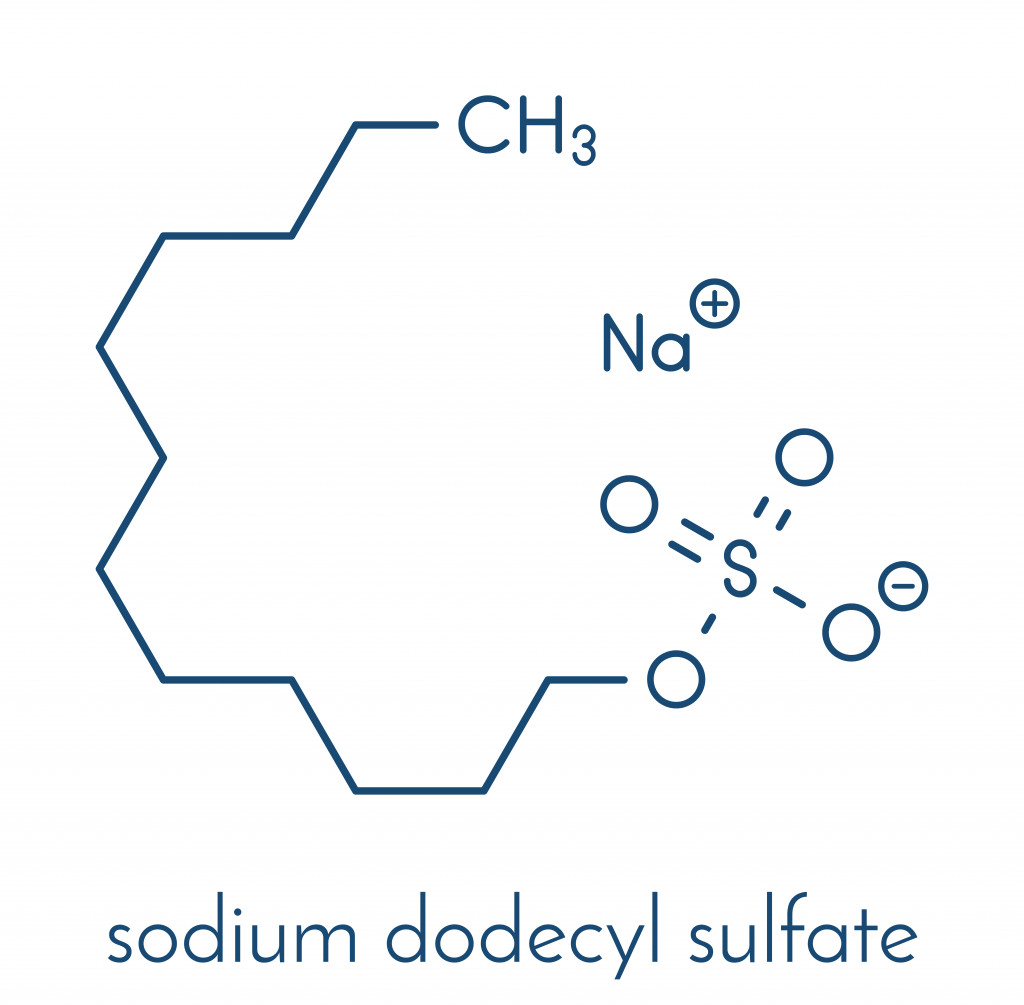
Sodium Laureth Sulfate (SLES) is a milder surfactant used in cleaning products. SLES has an extensive range of use and is present in household and personal care products.
A tweak in the chemical process of producing SLES decreases irritation, making it gentler on both the hair and skin. It doesn’t strip the skin of excess moisture, leaving it instead soft and nourished. SLES is one of the top choices for surfactants that act as foaming agents.
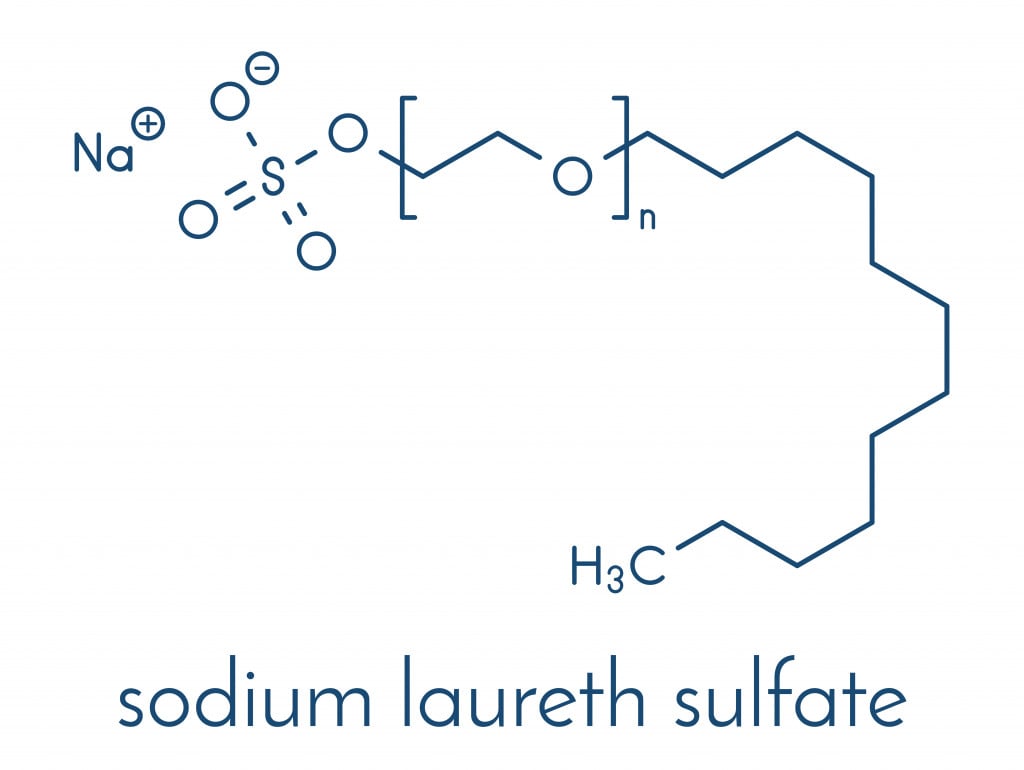
Why Are SLS And SLES Important In Cleaning Products Like Shampoo?
When you buy a shampoo, you expect it to efficiently remove dust, dead cells and oil from the scalp. SLS and SLES play a vital role in this process. Being a surfactant, they can pull out the dirt from the hair.
Surface active agents (also referred to as surfactants) are amphipathic molecules. They are comprised of two parts: a non-polar, hydrophobic tail (hydro=water, phobic=hating/repelling) and a polar, hydrophilic head (hydro=water, philic= love/attraction).
Dirt and oil tend to adhere to the substrate, like your scalp and hair. To successfully clean the scalp, the aqueous solution of shampoo must wet the hair and scalp. Here, a sulfate-based surfactant plays a critical role in the wetting and dirt removal. In a sense, they make water even wetter!
The hydrophilic head attaches to water and the hydrophobic tail attaches to the dirt, dust, oils, and dead skin. Because of its hydrophobic nature, the non-polar tail moves towards the surface of the water, carrying the dirt particle along with it, where it suspends itself in a gaseous phase. This allows the dirt and oil to wash away easily from the scalp and hair.
You might also enjoy the good lathery feel that you get when washing your hair. Again, you have sulfates to thank for that. We associate the effectiveness of shampoo with the amount of lather it produces. This is a vital psychological stimulus guided by visual and tactile perceptions. A good amount of foam is a significant cue for product quality and a sign that the shampoo is performing its intended task of cleaning our hair!
Woman washing her hair
Foaming has an important function in cleaning besides generating consumer appeal. According to a study, foam aids in the removal of oil and dirt from the surface. The lather suspends the dirt by creating greater surface tension in water. The increase in surface tension traps dirt and oil, making the removal easy during rinsing.
Even so, many believe that foam has no functional attribute in a product, other than the aesthetic purpose of cleaning.
Are Sulfate-based Shampoos Harmful?
SLS is an aggressive detergent that removes sebum from the scalp and hair, but it can also strip the hair of moisture. SLS is usually formulated with an anionic surfactant like cocomonoethanolamide (CMEA). It increases the foam’s stability and gives an improved after-feel to your hair. The main characteristic of SLS is that it is high foaming, has good solubility in water, and is entirely biodegradable.
SLES has good cleaning, emulsifying, wetting and foaming abilities, along with greater tolerance to hard water. It is also biodegradable and causes less skin irritation. Consequently, this makes sulfate the heart of most shampoos and other cosmetic formulations.
Recently, it seems that every chemical with a long name is being demonized and termed a carcinogen. The same is the case with SLS and SLES.
The amount of SLS found in cosmetic products ranges from 0.01% to 50%. People lean toward the belief that SLS causes skin and eye irritation, hair loss and even cancer, but these claims lack any scientific credibility.

At high concentrations, SLS can cause eye irritation, which is why it’s recommended to rinse your eye out if shampoo comes in contact. However, proper formulation and testing can limit irritation.
What Does The Research Say?
Neither SLS nor SLES cause skin irritation if used briefly and then rinsed off. Research shows that claims associating hair loss with SLS-containing products were unsubstantiated.
The same study also states that “there is no scientific evidence supporting the claims that SLS is carcinogenic.” SLS is not listed as a carcinogen by the International Agency for Research on Cancer (IARC), the U.S. National Toxicology Program, or the European Union. Speaking from an environmental perspective, SLS is a sustainable material. It is 100% bio-based content, with 99% biodegradability and a low potential for bioaccumulation.
Thus, the claims of SLS being toxic are not scientifically supported.
Similarly, the claims that SLES is toxic has confused some consumers. According to another study, at higher concentrations, SLES causes eye and skin irritation. However, the amount currently used in cosmetic products is safe, with no adverse effects on health.
Although SLES isn’t a carcinogen, a contaminant called 1,4-dioxane is a potential carcinogen in humans. 1,4-dioxane is formed when there are high levels of polyethylene glycol (PEG). PEG is a byproduct generated during the manufacturing of SLES.
Due to the risk of 1,4-dioxane being a possible carcinogen, many companies have started using 1,4-dioxane-free SLES. They do this by adding an extra purification step to the manufacturing of SLES.
Even so, there is no adequate evidence to confirm that 1,4-dioxane is a human carcinogen.
The cosmetic ingredient review expert panel has concluded that Sodium Laureth Sulfate is a safe cosmetic ingredient and their present concentration in products is non-irritating.
To sum it up, SLS and SLES can be irritating, but only at concentrations higher than currently being used. There is some limited proof of a connection between cancer and SLS or SLES-based shampoos, but many scientific studies have found the claims of SLS and SLES being toxic to be misleading.

Conclusion
If you are still concerned about skin and eye irritation and the possibility of cancer, “sulfate-free shampoo” is the way to go! Even though such concerns are unfounded and lack scientific evidence, the market has seen a rise in demand for such “sulfate-free” options.
Try going sulfate-free and decide for yourself which works better for you!
References (click to expand)
- Chemistry and Technology of Surfactants - Wiley. John Wiley & Sons, Inc.
- Cosmetic Formulation: Principles and Practice - 1st Edition. Routledge
- Broze G. (1999). Handbook of Detergents, Part A: Properties. Taylor & Francis
- Robinson, V. C., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Marks, J. G., … Andersen, F. A. (2010, May). Final Report of the Amended Safety Assessment of Sodium Laureth Sulfate and Related Salts of Sulfated Ethoxylated Alcohols. International Journal of Toxicology. SAGE Publications.
- What Makes Shampoo Foam? Everyday Compounds. Compound Interest
- Cornwell, P. A. (2017, December 14). A review of shampoo surfactant technology: consumer benefits, raw materials and recent developments. International Journal of Cosmetic Science. Wiley.
- Bondi, C. A. M., Marks, J. L., Wroblewski, L. B., Raatikainen, H. S., Lenox, S. R., & Gebhardt, K. E. (2015, January). Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products. Environmental Health Insights. SAGE Publications.