Table of Contents (click to expand)
Soaps, because of their chemical properties, such as being amphipathic, can break apart the cell membranes of bacteria and other pathogenic cells, as well as the envelope of many viruses.
Parents, doctors and even soap advertisements all advise us to wash our hands with soap after we come home and before we eat. Washing our hands with soap is a vital hygiene practice, especially during flu seasons. The reason behind this practice is the soap’s ability to “kill” germs.
It’s astonishing that a thing as mundane as soap can be our best defense against the deadliest of viruses. So, what gives soaps the ability to shield us against germs? And do they really “kill” the virus?
Recommended Video for you:
What Is Soap?

Right… but chemically speaking, soaps are salts of a fatty acid. They are made via the hydrolysis of fats and oils (triglycerides) from natural sources using a lye solution. Lye is an alkali made by preparing an aqueous solution of caustic soda. This process is known as saponification. The pH of soaps lies within the range of 9-10.
All soaps are essentially surfactants (surface-active agents) and are made of amphiphilic molecules. This means that they have two parts: a hydrophilic (water-loving) head and a hydrophobic (water-hating) tail. Surfactants are special chemical compounds that can modify surface tension between various phases.
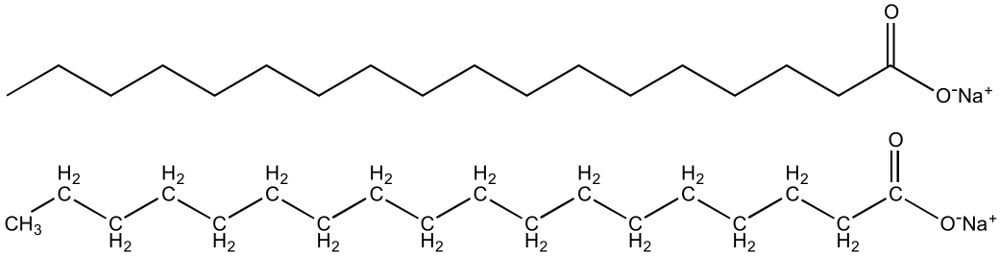
The carboxylate group of the soap forms the polar head, which is attracted towards water molecules. Meanwhile, the non-polar tail containing the aliphatic chain is repelled by water and has an affinity towards lipids and oils. This dual character of the soap allows it to dissolve both polar and non-polar molecules.
How Does Soap Work?
To understand how soaps destroy germs, you must first understand how they work under regular circumstances. With that in mind, let’s take a look at how soaps and detergents wash clothes so effectively.
Detergents can easily remove dirt and grease on clothes due to the action of the amphiphilic molecules. When you mix detergent into the water, the polar head orients itself towards the water phase. Whereas, in order to run away from water molecules, the hydrophobic tail orients itself towards non-polar molecules, such as oils.
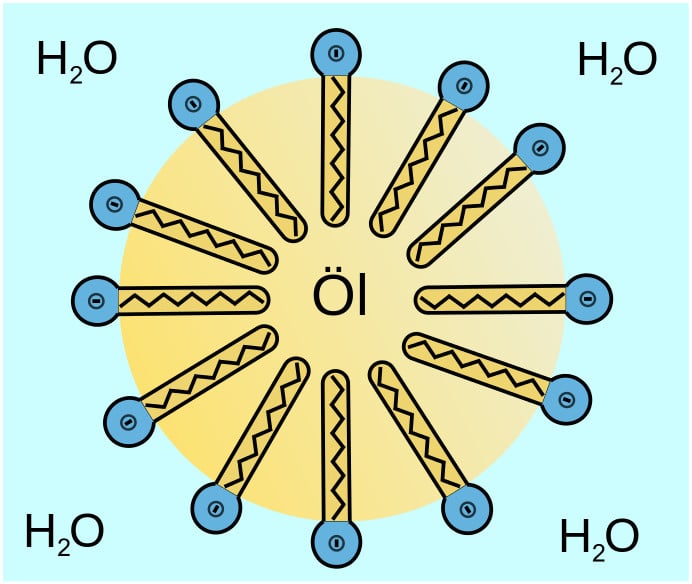
The hydrophobic tail attaches itself to the dirt and oil stains to prevent contact with water. At the same time, the hydrophilic head links itself to the water molecule. The soap then yanks the oil off the surface by orienting itself in a circular structure around oil particles, known as micelles. These micelles escort the oil molecules to the surface of the water. Here, the lather of the soap traps the dirt and oil, allowing it to rinse away with water.
However, what does this have to do with viruses?
How Do Soaps Break Down Viruses?
In a manner of speaking, viruses are similar to oil molecules. How, you ask? Well, the virus is essentially genetic material wrapped in a protective layer, called the envelope. These envelopes are made of fats or, scientifically speaking, lipid bilayers. They also contain a very integral part of the virus: protein spikes. These spike proteins latch on to the receptors present on the host cells, allowing the virus to infect them.
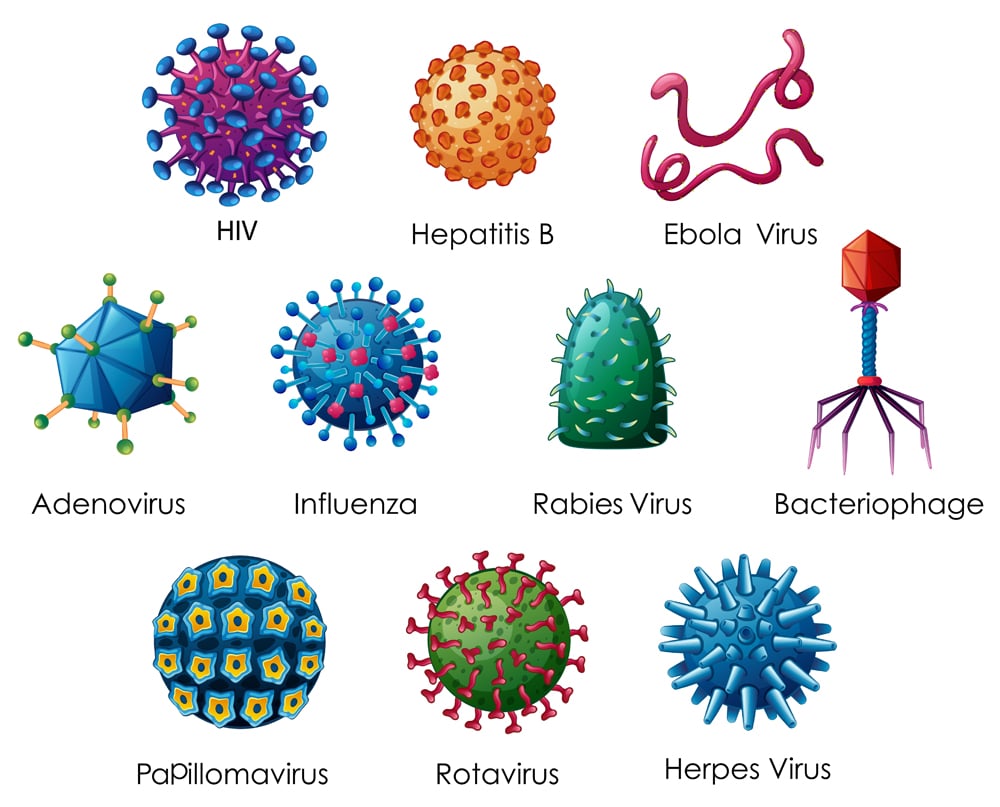
Viruses with a lipoprotein envelope include the Ebola virus, H1N1 influenza virus, herpes and the currently trending- coronavirus, just to name a few.
Just as in the case of washing grease stains off clothes, soap molecules attach themselves to the virus and other germs. When you wash your hands with soap and water, the hydrophobic tail of the soap starts looking for an area to get away from the water molecules. When they find the virus, the soap molecules start surrounding it. The hydrophobic tail sticks to the lipid bilayer wall of the virus and plucks it out from a given surface, such as your skin.
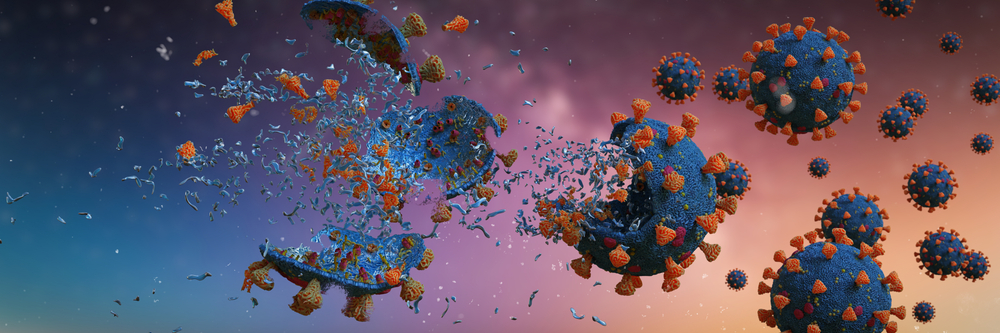
It doesn’t stop there. The hydrophobic tail further penetrates the virus, seeking to further get away from the water. Just like popping a bubble with a pin, the tail pops open the outer wall of the virus. This splits the virus apart, causing its contents to release into the soapy water. The remains of the virus rinse away when you wash your hands.
Do Soaps “Kill” The Virus?
Even though many soap advertisements claim that soaps “kill” germs, technically this is not true. Soaps simply tear the virus apart and remove it from the surface.
Think of this as the soap giving the virus a good rub-a-dub-dub. So much so that at the end of it, the virus still technically remains, but only in pieces, without cell walls and spike proteins. Also, if there is no cell wall holding the contents of the virus together, is that even really a living virus?
Conclusion
Contrary to what many believe, soaps are much more effective in killing germs than alcohol-based sanitizers. Don’t get me wrong, sanitizers do kill viruses and germs, but they cannot wash the virus away. Instead, they leave behind sterile dirt and dead viruses on your hands. Furthermore, since they are only 99.9% effective, they might also leave behind some active viruses on your skin.
Hand sanitizers can be a good substitute for soap and water. However, when possible, one must opt for soap and water to clean the hands. Health agencies like the CDC recommend washing our hands with soap and water for 20 seconds to successfully wash viruses and germs off your hands.
References (click to expand)
- How soap kills the COVID-19 virus - Queen's University Belfast. Queen's University Belfast
- Soaps or Santinizers The Great Debate - NISER. The National Institute of Science Education and Research
- . (2016). Soap Manufacturing Technology. []. Elsevier.
- How Soap Kills COVID-19 on Hands - Unesco.org. The United Nations Educational, Scientific and Cultural Organization
- SYNTHESIS AND PROPERTIES OF SOAP (FATS/OILS .... rsc.org