Table of Contents (click to expand)
The Schrodinger equation gives information about the quantum system under study by making use of complex mathematical formulations. It is the basis of quantum physics.
It is highly likely that you’ve come across the Schrodinger wave equation in your high school or college science classes. It is one of the most famous equations in quantum physics, and after learning as much as I have about quantum physics, I know one thing for sure: every topic of the quantum realm requires a gentle introduction.
So, let’s begin by understanding why the Schrodinger wave equation is such an important equation, as well as why it’s so famous in both scientific and pop culture communities.
Recommended Video for you:
Why Is The Schrodinger Wave Equation So Famous?
Before Schrodinger came up with this equation, the quantum physics community was unhappy with the mathematical formulation of the atomic model. The math was tedious and there was no room for visualizing or imagining a quantum system. These were just a few of the many problems that Schrodinger recognized.
When he formulated his legendary equation, it changed the face of quantum physics completely. It gave a huge amount of information about the quantum world and made visualizing it a lot easier.
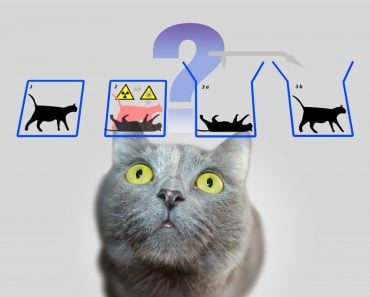
One of the results of this visualization is the very famous Schrodinger’s cat thought experiment.
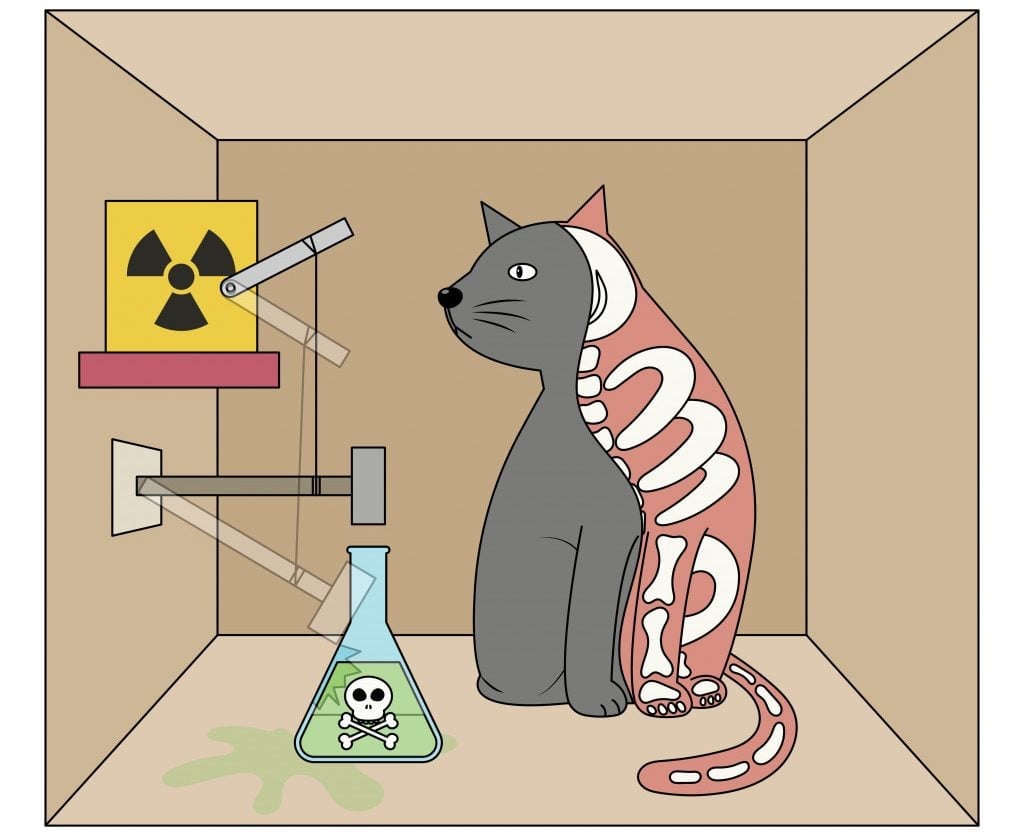
If you had come up with a thought experiment about a cat in a box alongside a radioactive substance and poison today, it would definitely garner some pointed stares, but despite its confusing fate, Schrodinger’s cat remains very famous.
The cat being simultaneously dead and alive not only triggered a deeper understanding of the quantum world, but also an awareness of the meaning of observation and consciousness in Physics.
The equation became the basis of understanding and formulating further quantum physics equations and won Schrodinger the 1933 Nobel Prize in Physics, alongside Paul Dirac.
Now that our introduction is complete, let’s call the equation itself to the stage.
Cue drumroll, pull curtains!
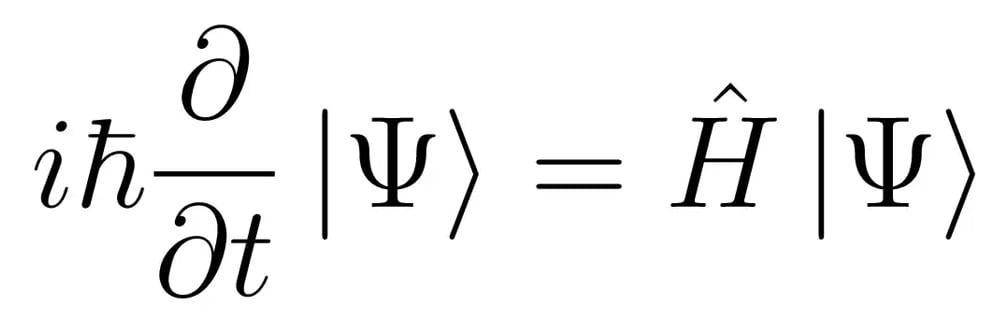
Confused? Don’t worry… I was too.
However, this is actually the simplest form of the Schrodinger equation, regardless of how much it may resemble alien script. Now bear with me… we will decode this alien script one term at a time.
First off, take a look at the  part of the equation. The terms definitely look strange, but are actually just constants.
part of the equation. The terms definitely look strange, but are actually just constants.  is called a complex or imaginary number, while
is called a complex or imaginary number, while  is Plank’s constant divided by
is Plank’s constant divided by  .
.
The next term  is the subject of this mammoth equation. And
is the subject of this mammoth equation. And  is famously called the wave function in quantum physics. It’s quite a celebrity in the quantum community!
is famously called the wave function in quantum physics. It’s quite a celebrity in the quantum community!
What Is A Wave Function?
In quantum physics, waves are what make up our universe at its most basic level. The particles are seen as physical manifestations of these interacting waves.
So, in order to study an object of the quantum world (a quantum object), we must study these waves.

Now, the mathematical formulae that give us information about quantum objects are what we call wave functions.
So, to put it in the simplest terms,  is the quantum system we are studying!
is the quantum system we are studying!
So, if we want to study a positively charged nucleus, that is our quantum system. Similarly, if we are studying an electron in a magnetic field, that will become our quantum system, and so on.
What Is A Quantum State?
Now, what does the strange-looking bracket  around the wave function,
around the wave function,  signify?
signify?
This tells us that we are dealing with the state of a quantum object, also known as the quantum state.
However, the state of an object in the quantum world is a bit tricky to understand.
For instance, in our macroscopic world, we can tell the position and momentum (states) of an object like a car or a chair at any given time with conviction and certainty.
But when it comes to objects in the quantum world, like electrons or neutrons, we can only talk about their states in terms of probability. The tricky part is that there can be many possible states of a given quantum system.
For instance, a particle may have the probability of existing in two different places at the same time until an observer makes a reading! This very strange fact is what Schrodinger used as the basis for his famous cat thought experiment. One interpretation of this fact about multiple probabilities is the multiple universe theory, but that’s a story for another day!
Coming back to the quantum state,  , which is the subject of Schrodinger’s equation, we need to see what the equation is doing to it.
, which is the subject of Schrodinger’s equation, we need to see what the equation is doing to it.
Take a look at the  term before this quantum state. This term signifies that we are trying to find the change in the quantum state of an object with time. Or rather, we are attempting to predict the future of the quantum state.
term before this quantum state. This term signifies that we are trying to find the change in the quantum state of an object with time. Or rather, we are attempting to predict the future of the quantum state.
Basically, the Schrodinger wave equation is trying to predict the change in the quantum state of a given quantum system!
Hamiltonian
Now, let’s come to the last part of the equation –  . This H is called Hamiltonian, and the cap symbol above it represents that this is an operator. So
. This H is called Hamiltonian, and the cap symbol above it represents that this is an operator. So  is the Hamiltonian operator.
is the Hamiltonian operator.
This operator is the whole reason why Schrodinger came up with this equation. Previously, the matrix formulation had been used, which was very inconvenient and considered one of the greatest problems of quantum mechanics. Schrodinger instead came up with the Hamiltonian operator, which gives the total energy of the quantum system.
Simply put, the Hamiltonian operator acts or operates on the given quantum state to determine the total energy of the system!
Now that we have all the pieces of the puzzle, let’s put them together to see what is really happening in the Schrodinger wave equation.
The quantum state of a given quantum system under study is changing with time. This change depends on the total energy of the system. Basically, with this equation, we are trying to predict the future of a given quantum system!
This was a general and introductory look at the Schrodinger equation, but there is far more to this world if you’re curious.
At this point, I can safely say goodbye and send you off to greener pastures, or nudge you towards the deeper end of the quantum pool!