Table of Contents (click to expand)
To convert grams to moles, you need to find the molecular mass of the compound and divide the number of grams of the compound by its molecular mass.
Converting grams to moles involves 2 steps:
Step 1: Find the molecular mass of the compound.
Step 2: Divide the number of grams of the compound by its molecular mass.
In order to determine the number of moles of a given compound, the first thing you need to do is find the molecular mass (or molecular weight) of the compound in question. Once you’ve done that, you need to determine how much the compound weighs physically (in grams). After you get both of these values, you need to divide the physical weight of the compound by its molecular weight.
Before we delve deeper into the grams to moles conversion calculation procedure, let’s do a quick recap of the basics.
Recommended Video for you:
What Is A Mole In Chemistry?
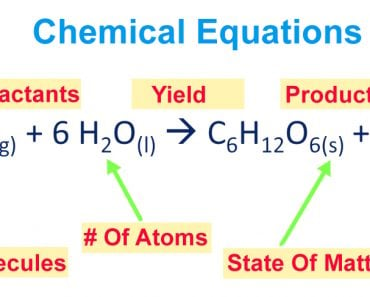
A mole of a substance is defined as the mass of substance containing the same number of fundamental units as there are atoms in exactly 12 grams of Carbon-12, which is approximately 6.022 x 1023 atoms.
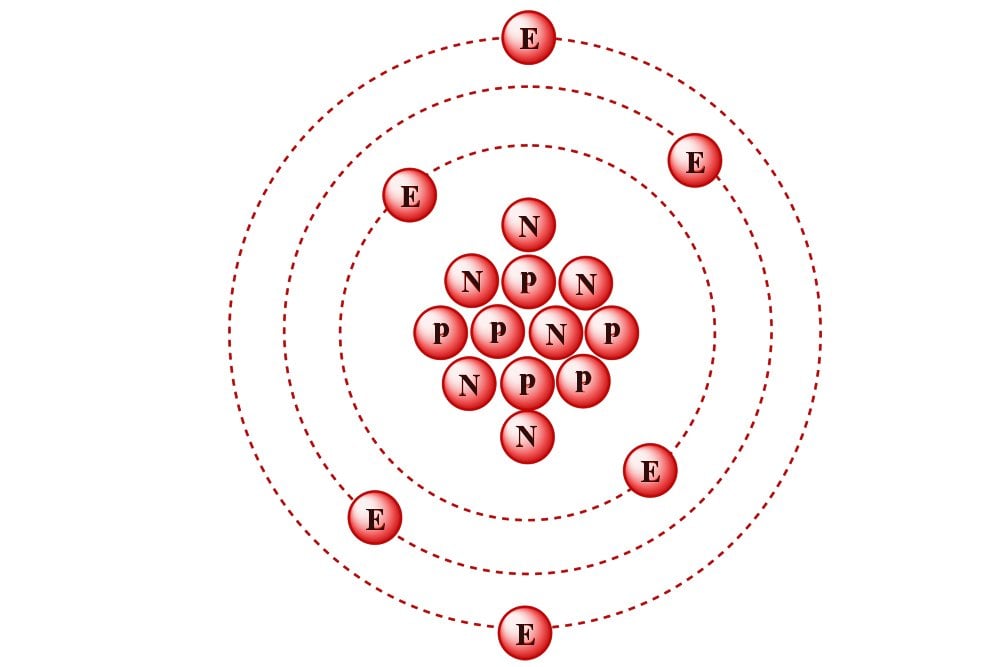
I also want to state an easier, simpler-to-understand definition of a mole (of chemistry).
Moles are a unit of measurement of chemical compounds. Just like how physical quantities, such as distance, mass, time etc. have measurement units (meters, grams, seconds respectively), similarly, chemicals are measured in moles. In short, you could say that a “mole” is the unit of amount in chemistry, i.e., it tells you how much of a particular chemical is present.
Note that the mole in chemistry should not be confused with the mole related to the human body. While the former is a unit of measurement, the latter is a dark spot on the skin comprised of skin cells that have grown in a group.

When chemists perform a chemical reaction in a laboratory, they want to ensure that they are using the right amounts of all chemicals involved in the reaction before actually starting the process. Just as it’s much simpler and more convenient to measure intergalactic distances in light-years instead of centimeters, it’s easier to count atoms in moles rather than counting them in billions and trillions.
Here’s an interesting thing about moles: 1 mole of any element or chemical is always the same number. For instance, 1 mole of hydrogen and 1 mole of oxygen both contain approximately 6.022 x 1023 atoms. The respective masses of hydrogen and oxygen, however, are different.
The same applies in the case of chemical compounds. No matter what their molecular masses are, all of them contain the same number of molecules for a particular number of moles, i.e., 1 mole of glucose contains the same number of molecules as 1 mole of methane. Their masses, however, are different.
Now that we understand what a mole is, it’s time to proceed and look at how to determine the number of moles present in a chemical compound, provided that we already know the physical weight of the compound in question.
Grams To Moles Conversion Formula
The first step is to determine the molecular weight (or molecular mass) of the compound you’re dealing with. A compound is composed of a number of atoms of different elements, and their combined weight becomes the molecular weight of the compound.
Let’s consider the example of NaCl (sodium chloride or common salt) to understand this concept better.
NaCl is made of two atoms: sodium (Na) and chloride (Cl). The atomic mass of sodium is 22.98, while the atomic mass of chlorine is 35.543.
Molecular mass of NaCl = atomic mass of sodium + atomic mass of chlorine
Thus, the molecular mass of NaCl turns out to be 58.52 grams/mol.
This is how you can determine the molecular mass of any compound.
Next, you need to find the ‘physical’ weight of the compound in question, which, in this case, is sodium chloride. You can measure the weight of your sample using an analytical balance (also called a “lab balance”), a device that weighs substances in grams. Most chemistry laboratories have this device on hand to measure the weight of chemicals.

Let’s assume, in this case, that we have a 100-gram sample of common salt.
Once you have both the molecular mass and physical weight of the compound, you can use a simple formula to calculate the number of moles present in the sample.
Number of moles = Weight of compound (in grams) / molecular weight of compound
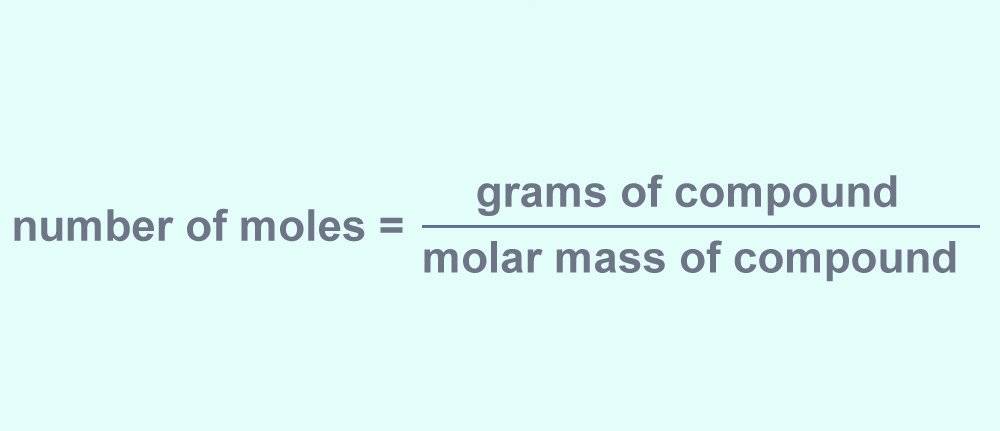
In our example, the weight of NaCl is 100 grams, and its molecular weight is 58.52 g/moles. Thus, the number of moles in the given sample of NaCl comes out to be 1.70 moles (100/58.52).
Let’s take another example – a sample of 50 grams of water (H2O).
The molecular mass of water is 18 g/mol.
Therefore, the number of moles in this sample of water comes out to be 2.78 moles (50/18).
Using this formula, you can determine the number of moles present in a given sample of any compound.