Table of Contents (click to expand)
Scientists experimented on and observed elements and their behavior, which helped them figure out the existence of atoms and frame the atomic theory.
The atomic theory came into being long before the first transmission electron microscope, which means that we knew about atoms long before we saw them!
Who ever said you need to see things to know about them! We haven’t seen gravity, or electricity, or even magnetism and yet we know a great deal about these things. Science has always been kind of weird in the sense that things known to us are not solely available through visual observation. Curiosity drives all scientific breakthroughs, but when people lacked enough physical and tangible proof, they prove their theories through indirect results. This method of determining results is the foundation of the modern atomic theory.
Recommended Video for you:
Why Can’t We See Atoms?
Quite simply, because they’re so incredibly small! An object is visible when it deflects the light falling on it. The size of atoms falls between 30-300pm, which is approximately of the order 10-12m. For optical microscopes, atoms are invisible, i.e., atoms do not interact with the light particles, so there is no deflection. It wasn’t until the invention of electron microscopes that we first got a glimpse of the atom. An electron beam, which has a lower wavelength than visible light, is scattered when it hits the target; this scattering allows for the creation of an image. There are many more advanced microscopes that not only allow us to observe atoms, but also aid in moving atoms around in a sample to study them!
How Did We Think Up The Existence Of An Atom?
Atomic theory formulation spans many years. Here is a rudimentary attempt to timeline the process of atomic theory formulation, which started in the pre-Socratic period.
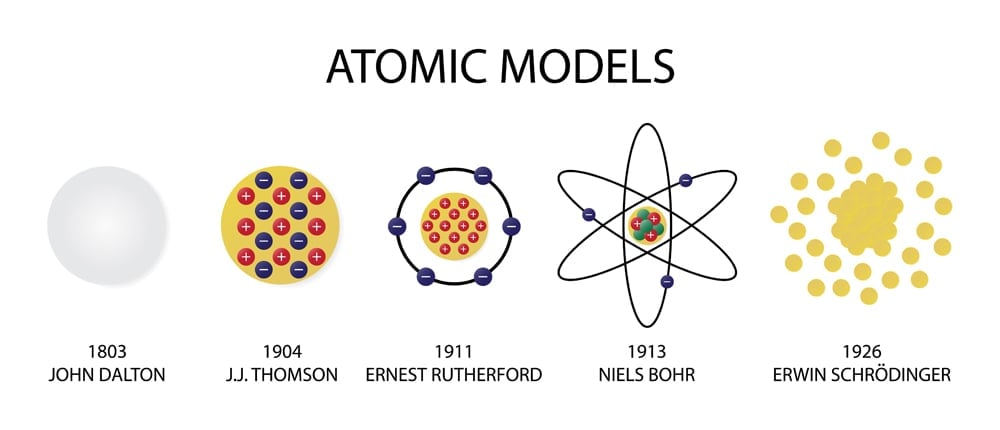
- Democritus, the Greek philosopher, was the first to think of atoms. If a piece of matter is divided into smaller and smaller parts, then all the particles still possess the same properties. If we go on dividing matter, there comes a time when we cannot divide it further. This indivisible particle is ‘atomos’. One notable thing was that atomists during this time believed that atoms of all matter were similar in all respects.
- The atomist teachings were lost for approximately two millennia until the 1800s, when John Dalton first proposed an atomic theory. While trying to figure out why elements only combined in specific ‘whole number ratios’ (like 1:2 or 3:4 etc.), Dalton determined that there must be an indivisible solid, mass-bearing and indivisible particle that is unique for each element. He believed that because this tiny particle was indivisible, compounds could not combine in fractional ratios.
- J J Thompson’s ‘plum pudding model’ in the late 19th century was the first model to break the myth of the atom being a solid particle. The cathode ray tube experiment that first discovered electrons led to a modification of the atomic model. The new model was not a solid ball, but had negative charge floating in a sea of positive charge. (It was a positively charged sea because atom as a whole were known to be neutral)
- Ernest Rutherford’s gold foil experiment further acknowledged the fact that the positive charge was only contained in a small part of the atom. Because most alpha rays passed without deflection, the atom had to be largely empty; the few rays that got deflected had likely hit the nucleus. The plum pudding model was replaced by Rutherford’s “nuclear model”, but the position of electrons was still disputed.
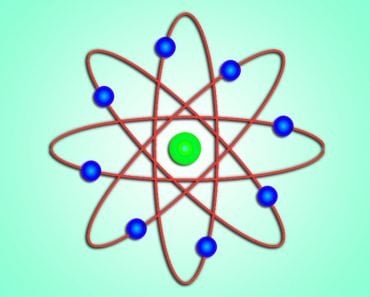
- The early 20th century saw the rise of quantum mechanics. Max Planck and Einstein, the pioneers of quantum mechanics, explained that anything that is quantized is allowed to take up only specific values. Niels Bohr, a Danish scientist strongly believed that the structure of an atom was similar to the planetary model. Bohr used the theory of quantization to explain how electrons stay in their orbits, despite orbiting the nucleus and thus not falling into the nucleus.
- Erwin Schrödinger’s discovery of the dual particle and wave behavior of electrons contradicted Bohr’s assumption of electrons being in specific orbits. We now have a quantum mechanical model of atom that calculates the probability of finding an electron in a region. It contradicts Bohr’s assumption of electron orbits having specific energy levels.

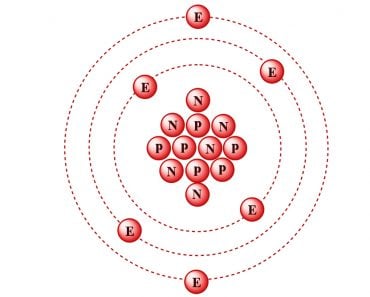
- While the model may seem complete, the mass of the nucleus was still a mystery. Although we knew about protons and electrons, scientists found that the nucleus weighed more than the combined weight of all the protons—almost twice as much! The discovery of neutrons (whose mass is very similar to protons) in 1932 by Chadwick helped to complete the modern atomic model. The atomic mass of the nucleus was now justified by the presence of these newly discovered neutrons.
As you can see, the modern atomic model is the result of many different observations, questions and experiments. If you observe the way the model has evolved over the years, it becomes clear that due to the lack of visual data available for scientists to analyze, they largely relied on experimental evidence. Remember, this was all way before the first transmission electron microscope first came into being!
Are There Any Other Subatomic Particles?
Modern microscopes, like electron beam microscopes and scanning probe microscopes, have helped us observe the structure of atoms and nano-particles… but there’s more!
Scientists at Stanford University built an electron accelerator that was designed to further nuclear research. Beams of electrons were accelerated at energies of 20 billion electron volts. When such a high-energy beam was targeted at liquid hydrogen and deuterium, researchers observed that electrons began scattering at wider angles and more frequently than anticipated. By the 1970s, it was realized that there are three scattering centers in the nucleus that cause the scattering pattern. This discovery was the first evidence of the existence of quarks!
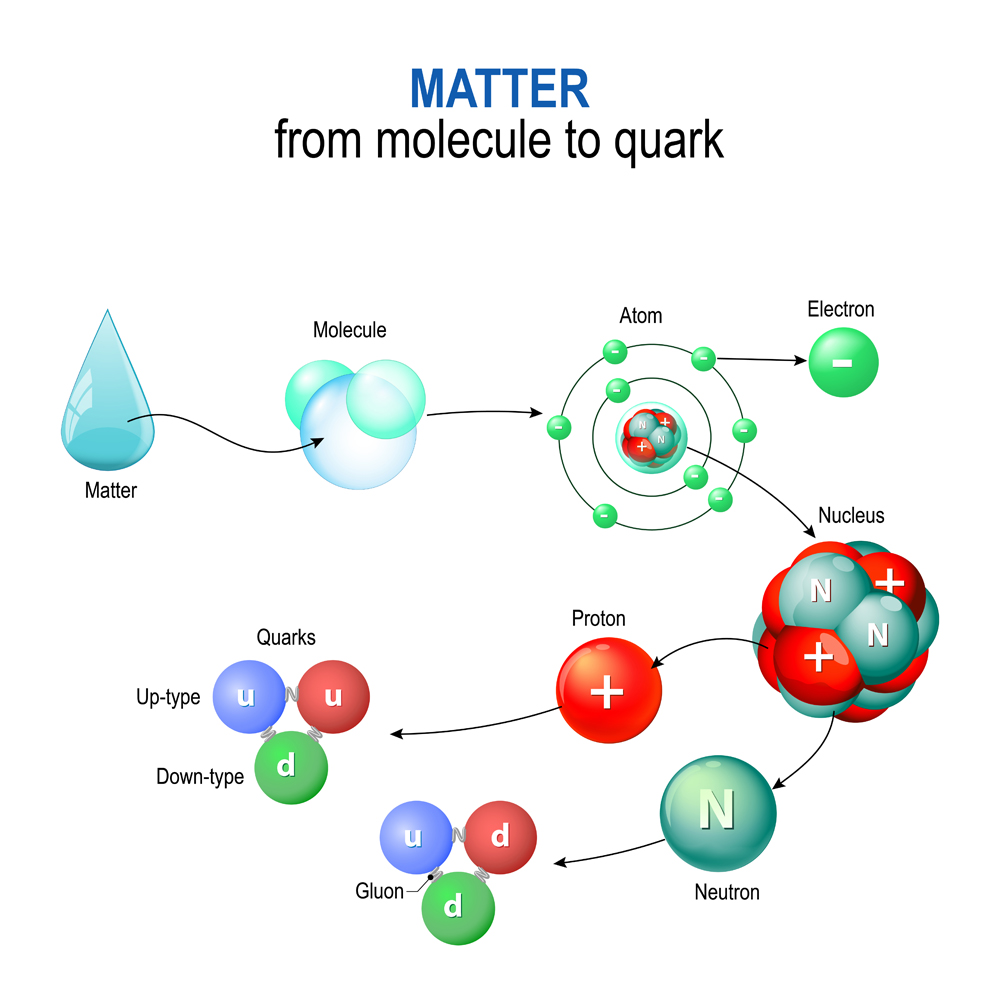
Until recently, we thought that electrons, protons and neutrons were the most fundamental subatomic particles, meaning that they were indivisible. However, quarks are the actual elemental indivisible particles that make up protons and neutrons! Electrons, for now, are thought to be indivisible, but I wouldn’t be surprised if there’s more to them!
The discovery of the atom and the subsequent discovery of subatomic particles proves the importance of observation and experimentation. The 20th century did not have powerful microscopes to provide much needed visual reference, and yet scientists were able to study atoms! Looking at the current trend of technology, we will have access to far more sophisticated apparatus in the future that will aid us in moving forward by leaps and bounds—into the very heart of the quantum realm and the far-flung corners of the universe!
References (click to expand)
- Did the Greeks discover atoms?. lanl.gov
- Questions and Answers - If there is no way in the world to see .... Thomas Jefferson National Accelerator Facility
- Quarks - Hyperphysics. Georgia State University
- Baggott J. E. (2017). Mass: The Quest to Understand Matter from Greek Atoms to Quantum Fields. Oxford University Press
- Seeing atoms - Science Learning Hub. sciencelearn.org.nz