Table of Contents (click to expand)
Light bulbs have been around for quite a while now, but you may not know that they work on complex principles of electrodynamics and thermodynamics.
Before the era of electrical lighting, it was quite a task to summon bright and long-lasting lighting. The only options available were candles and oil lamps, which were not very efficient at doing their job.
Conceived and patented by Thomas Alva Edison in the 1800s, incandescent light bulbs have continued to light up our world for generations.

Incandescent light bulbs have been such a hit that the original technology hasn’t undergone any drastic changes. It’s interesting to note that such a minute (but also critical!) part of our existence is based on important aspects of physics.
In this article, we’ll find the answers to some general questions regarding incandescent light bulbs.
Recommended Video for you:
How Is Light Produced In Light Bulbs?
An incandescent bulb mainly consists of two parts—the bulb and the filament.
The bulb is generally made of glass, within which is a vacuum. The vacuum helps in extending the life of the light bulb; if air particles are present inside the bulb, it will heat up quickly and the glass will break easily.
The filament inside the bulb is where the actual light is produced. It is made of a long and coiled material that is a good conductor of electricity, such as tungsten. Sometimes, the inside of the bulb is also filled with an inert gas, like argon. Inert gases help in slowing the process of tungsten filaments wearing out.
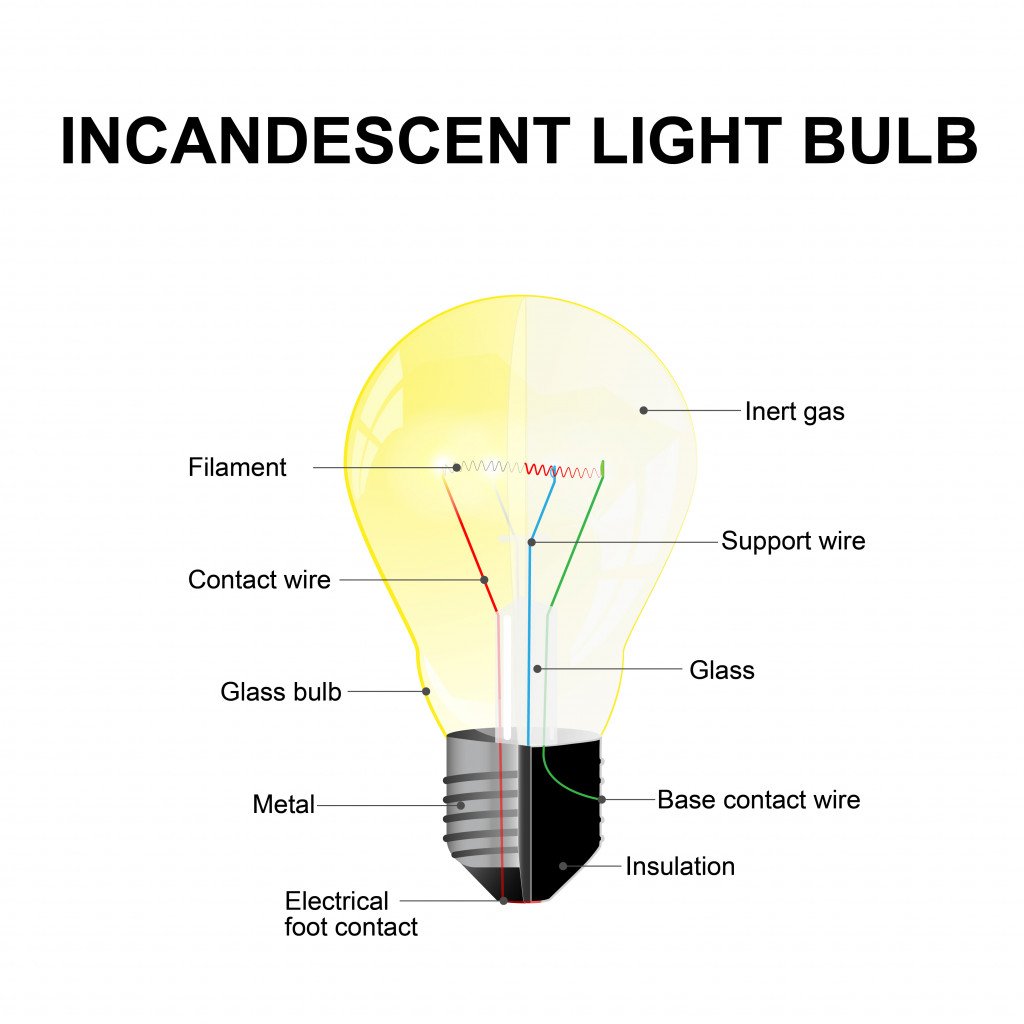
The filament is attached to metal contacts that are hooked to a power supply so electricity can flow through it.
When the electric current flows through the filament, the atoms are agitated and the electrons within them are excited to higher energy levels as soon as they absorb energy from the flowing current. The time that the electrons remain in this excited state is very small, and when they return to their original energy levels, extra energy is released in the form of photons (small packets of light energy).
In this way, the light bulbs glow!
Why Do Light Bulbs Get Hot?
One important point to consider here is that the filament of the light bulbs burns itself to produce light. This means that the electric current flowing through the filament heats it to the level where it starts emitting photons. The agitation and vibration of the atoms within the material of the filament produces heat energy.
Most of the electric current flowing through the light bulb is used for agitating the atoms. This generates heat energy, but only a small fraction of this electrical energy gets converted into light.
Also, the inside of the bulb is not completely vacuum-sealed and air particles conduct heat energy to the glass. The inert gas inside the bulb also conducts heat energy to the glass. Thus, when you touch a light bulb that is glowing for a long while, it’s hot.

For this reason, the filament eventually breaks, so incandescent bulbs don’t last long.
Hence, we can say that an incandescent light bulb is not particularly efficient in converting electrical energy into light energy, wasting energy in the form of heat.
Say Hello To The Brighter Competitors!
Due to humankind’s perpetual thirst for better options, the incandescent light bulb now has better competitors—the halogen bulb, fluorescent bulb, and LED (Light-emitting diode) bulb.
These different kinds of bulbs work on different mechanisms where the wastage of energy as heat energy is less and a more significant part of electrical energy is converted into light energy. They are economical, long-lasting, and more energy-efficient.
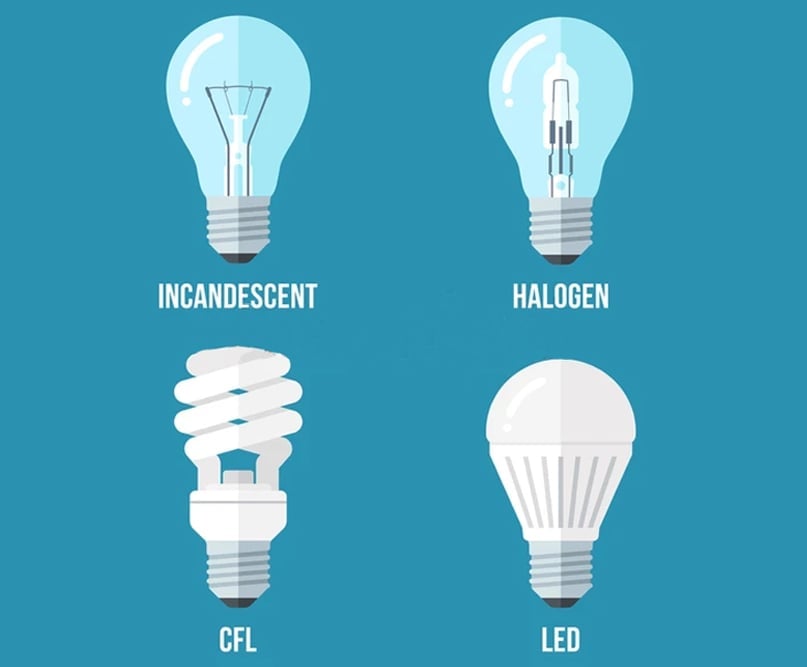
Halogen bulbs are an advanced version of incandescent bulbs where the tungsten filament is enclosed within a bulb-shaped quartz capsule, filled with a mixture of inert gas and a tiny amount of halogens, such as iodine or bromine. The “halogen cycle” redeposits the tungsten particles into the filament, allowing them to be reused and effectively elongating the life-span of the bulb.
Fluorescent bulbs use the principle of fluorescence, where the mercury vapor is energized using the electric current flowing through the bulb. This energized mercury vapor emits ultraviolet radiation to the phosphor coating on the inner walls of the bulb, causing it to emit light energy. It’s approximately four times more efficient and ten times more long-lasting than incandescent light bulbs.
LED bulbs emit light energy when they are forward-biased. They are made of LEDs, which are composed of semiconductor material. They are the most energy-efficient option on the market.
To know more about the mechanism of an LED bulb, check out the article ‘Why are LED lights so energy-efficient?’
Towards An Energy-efficient Future
With the depletion of energy resources, the world is trying its best to conserve them and shift to more sustainable solutions. The best artificial lighting option that one can opt for is LED lighting, due to the many advantages it has over all the other traditional lighting methods. LED light bulbs emit negligible heat energy, last up to 25,000 hours, and are available in a variety of colors.

The future of household and commercial lighting is very bright, thanks to humanity’s brilliant inventors, who are always on the hunt for new technologies!