Table of Contents (click to expand)
The limiting reagent (or reactant) in a reaction is found by calculating the amount of product produced by each reactant. The reactant that produces the least amount of product is the limiting reactant.
There are many things that need to go right for a chemical reaction to yield useful products: from the environment surrounding the reaction to the amount of the reactants present. Only once in a blue moon do all the reactants get converted into products.
In most reactions, one reagent (reagent and reactant are used interchangeably) is entirely depleted, while some quantity of the other reagents stays available for further reaction.
Since one of the reactants is not always available, the reaction hits a roadblock and does not continue. This reactant that gets completely used up, and thus limits the reaction from advancing forward, is called the limiting reactant or limiting reagent.

Recommended Video for you:
What Is Limiting Reactant?
Based on their amounts and their roles, we classify reactants into two kinds: limiting reactants and excess reactants.
Limiting reactants are those that get completely utilized in a reaction first and thus limit the amount of product that will be produced. Excess reactants, on the other hand, are the reactants that are still present after the reaction has reached a standstill.
Let’s say that you’re standing in a queue at your favorite bagel vendor. The bagel guy comes out of the cart and announces that he only has 10 bagels left. Now, you look up from your phone and start counting the number of people ahead of you in the queue. You count a total of 20 people, which is 10 more than the number of bagels that remain.
In our example, the customers standing in the queue and the bagels are reactants, coming together to produce happy-fed individuals (end product).
However, the number of bagels will limit the number of happy-fed customers that can be achieved in the end, making it the limiting factor (agent), while the customers will be considered the excess reactants.
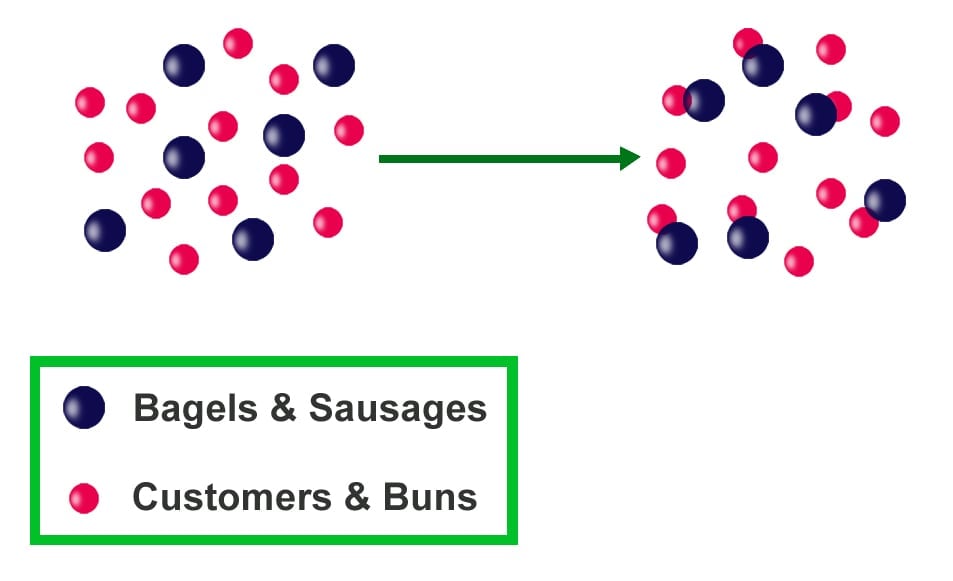
Another example is the number of buns and sausages needed for making a hot dog. It takes both a bun and a sausage to make a hot dog. An unequal number of the items (reactants) will result in an inadequate number of hot dogs (product). With 10 buns and 5 sausages, we will only be able to produce 5 hot dogs and will have 5 buns remaining. The buns are available in excess, while sausages are the limiting agent, controlling the number of hot dogs that can be made.
How To Find The Limiting Reactant?
There are a couple of ways by which one can determine the limiting and excess reactants in a reaction. However, the methods come with a prerequisite, which is to have a balanced chemical equation in hand.
The methods use stoichiometric coefficients from the balanced chemical equation to calculate ratios; if the coefficients themselves are incorrect, the final answers obtained will also be incorrect.
In the first method, we will find and compare the mole ratios of the reactants, while in the other one, we will find the amount of product that will be produced by each reactant. The one that produces the least amount of the end product is the limiting reagent.
Method 1: Using Mole Ratios
Let’s apply this method to the reaction of ammonia (NH3) and molecular oxygen (O2) to figure out the limiting reactant of the two. The reaction between NH3 and O2 yields NO (nitric oxide) and H2O (water). The balanced chemical equation for the reaction is:
4NH3 + 5O2 → 4NO + 6H2O
The equation is read as 4 moles of ammonia require 5 moles of oxygen to produce 4 moles of nitric acid and 6 moles of water.
We start by finding the stoichiometric ratio of the reactants. To do so, we simply divide the stoichiometric coefficients of each reactant. For ammonia, the stoichiometric coefficient is 4, while for oxygen it is 5.
Stoichiometric Ratio = 4 moles of ammonia / 5 moles of oxygen = 0.8 moles of ammonia/1 mole of oxygen
This implies that we need 0.8 moles of ammonia for each mole of oxygen.
Next, we find the mole ratios of the reactants. In most scenarios, the quantities of the reactants will be provided in grams. We convert the gram values to moles and then continue towards finding the limiting reactant. Let’s assume that we have 100 grams of ammonia and oxygen each. To convert this into moles, we divide these values by their molecular masses.
For ammonia, the number of moles = (100 g) / (17.04 g/mol) = 5.86 mol
For oxygen, the number of moles = (100 g) / (32 g/mol) = 3.125 mol
We can continue forward in two ways. One, by assuming a limiting reactant and finding the number of moles of other reactants required, or the other, by finding the actual ratio and drawing a conclusion from its value. Let’s go down the first path and assume ammonia to be the limiting reactant.
Assuming ammonia is consumed first, (5.86 moles of ammonia) / Stoichiometric Ratio = 4.688 moles of oxygen are required.

But we only have 3.125 moles of oxygen available for the reaction, so we will run out of oxygen before ammonia. Therefore, oxygen is the limiting reactant and ammonia is available in excess.
Method 2: By Comparing The Amount Of Product Produced By Each Reactant
Just as in the previous method, we start off with a balanced chemical equation and continue by determining the number of moles of each reactant. We will make use of the same values as those found in the previous method.
The number of moles of ammonia = 5.86 mol & the number of moles of oxygen = 3.125 mol.
Now we find the amount of the end product (NO) that each reactant will produce.
For ammonia, the mol of nitric oxide (NO) = Moles of NH3 available × Stoichiometric coefficient of NO/ stoichiometric coefficient of NH3 = 5.86 × 4/4 = 5.86 mol of NO
For oxygen, the mol of nitric oxide (NO) = Moles of O2 available × Stoichiometric coefficient of NO/ stoichiometric coefficient of O2 = 3.125 × 4/5 = 2.5 mol of NO
Since the amount of product produced by oxygen is less than that produced by ammonia, oxygen is the limiting reactant and ammonia is in excess.
Final Words
As we can see, the limiting reagent or limiting reactant in a reaction is the reactant that gets completely exhausted and thus prevents the reaction from continuing forward. It also determines the amount of the final product that will be produced.
Finding the limiting reactant is an important step in finding the percentage yield of the reaction. The percentage yield of a reaction is the ratio of its actual yield to its theoretical yield times 100. Theoretical yield is the yield predicted by stoichiometric calculations, assuming the limiting reactant reacts completely.
In simpler words, it is the amount of product produced from the limiting reactant. In our case, the limiting reactant is oxygen and the amount of product (NO) produced from it is 2.5 moles. Thus, the theoretical yield for the reaction is 2.5 moles.
The actual yield is the amount of end product obtained upon experimentation. Let’s assume the actual yield we obtained on experimentation as 2 moles. The percentage yield will then be:
Percentage yield = (Actual yield/Theoretical yield) × 100 = (2/2.5) × 100 = 80%
The theoretical yield calculated is usually higher than the actual yield that is produced. This happens due to a variety of reasons, including the reversible nature of reactions, the production of undesired by-products, errors in purification processes, etc.
References (click to expand)
- Limiting Reactant - The reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when all of the limiting reactant is consumed. - www.chem.tamu.edu
- Reactants, Products and Leftovers - Chemical Reactions.
- limiting reagents, theoretical , actual and percent yields.