Table of Contents (click to expand)
Carbon dioxide (CO2) is nonpolar because it has a linear, symmetrical structure, with 2 oxygen atoms of equal electronegativity pulling the electron density from carbon at an angle of 180 degrees from either direction. Polarity in a molecule occurs due to the unequal sharing of valence electrons; since there’s no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar.
However, before we get to the bottom of this, it helps to first understand a few underlying concepts regarding the polarity of a molecule.
Recommended Video for you:
What Is Polarity?
Molecules that have regions of positive and negative charge are referred to as ‘polar’, and this property of such molecules is called polarity.
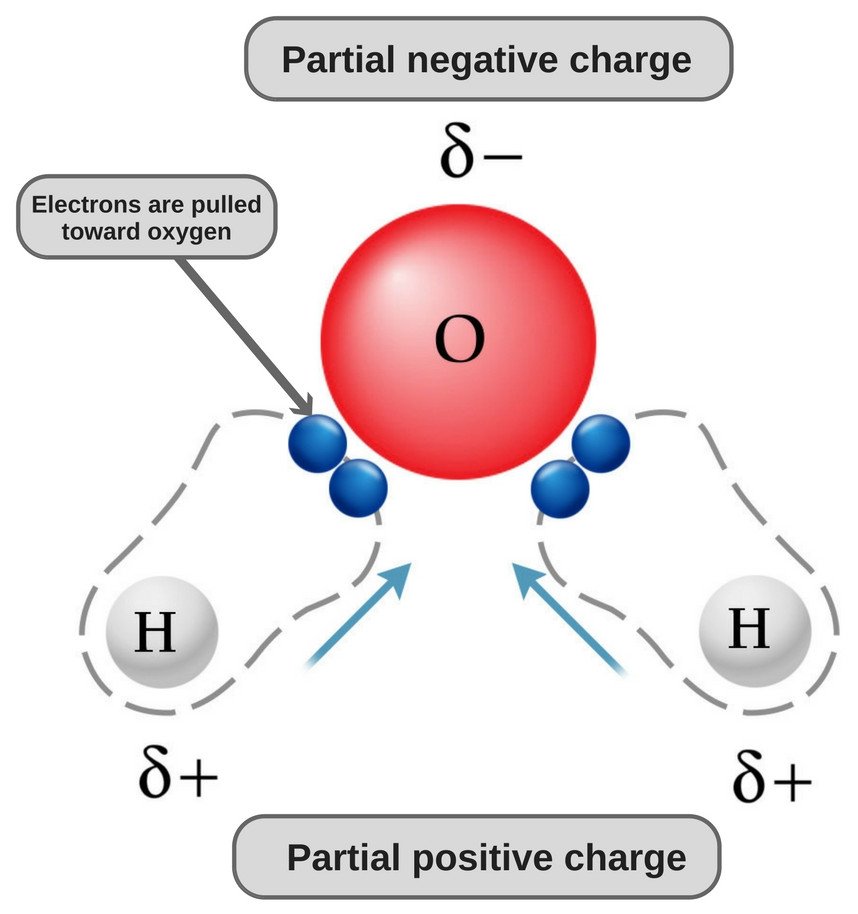
Take water, for instance. Due to its bent structure and the type of bonds it has, one end of its molecule (i.e. the oxygen end) possess a slight negative charge, while the other end possesses a slight positive charge (i.e., the hydrogen end). This makes water a polar molecule.
 Similarly, molecules that do not have regions of positive and negative charge are referred to as nonpolar. Ethane, for example, is a nonpolar molecule. The shape that it has and the type of bonds it consists of leave it with no regions of charge.
Similarly, molecules that do not have regions of positive and negative charge are referred to as nonpolar. Ethane, for example, is a nonpolar molecule. The shape that it has and the type of bonds it consists of leave it with no regions of charge.
There’s a notion in chemistry that says ‘likes dissolves likes’; this is actually a reference to a substance’s solubility in another. Polar materials tend to be more soluble in polar solvents, and the same is true for nonpolar materials.
What Makes A Molecule Polar?
The polarity of a molecule is related to the shifting of electrons in a particular direction. This, in turn, depends on the polarity of the bonds present in the molecule, as these bonds also contain electrons.
The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons won’t be able to shift in any direction. On the other hand, if the two atoms are different, they will definitely have dissimilar powers to attract the electrons of the bond.
Hence, the atom with the higher power to attract electrons towards itself (i.e. it’s more electronegative than the other atom), will acquire a slight negative charge on itself, and the bond between the two atoms will become polar.
All in all, you could say that the electron density of a polar bond accumulates towards one end of the bond, which results in that end possessing a slight negative charge, while the other end has a slight positive charge. This makes a molecule polar. Likewise, if a molecule does not have regions of positive and negative charge, it’s considered nonpolar.
However, an interesting thing to note is that the larger the electronegativity difference, the more polar the bond will be within a molecule. Carbonyl compounds are polar because the carbonyl carbon is slightly positive. So, shouldn’t carbon dioxide, which contains a positive carbon and two partially negative oxygens, be polar?
Why Is Carbon Dioxide Nonpolar?
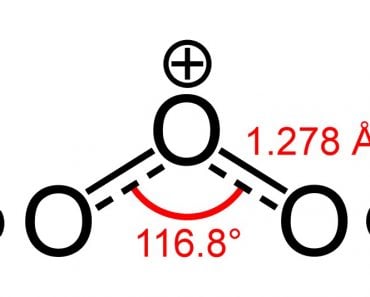
If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. Let’s look at the structure of carbon dioxide:
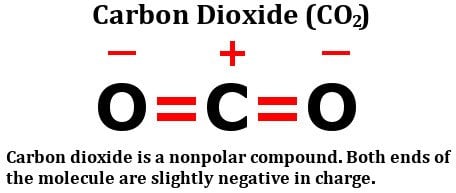
As you can clearly see, the molecule has a carbon atom sharing two double bonds with oxygen. Sure enough, oxygen is more electronegative than carbon, so, one might think that the electrons present in the bond between carbon and oxygen would be pulled towards the oxygen atom.
However, that doesn’t really happen. The reason lies in the geometry of the molecule. As you can see, both of these double bonds are at 180 degrees from the central carbon atom. Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force.
The result is that there is no net shifting of electrons in any direction, so there is no build up of net charges on any of the atoms, making the carbon dioxide molecule nonpolar.
References (click to expand)
- Biology Topics | Principles of Chemical Science | Chemistry. MIT OpenCourseWare
- Main Experiment Menu - Harper College. William Rainey Harper College
- Molecular Polarity - chemistry.bd.psu.edu:80
- Molecular Polarity. The University of Wisconsin–Madison
- The Polarity of Molecules - archives.library.illinois.edu
- Biology Topics | Principles of Chemical Science | Chemistry. MIT OpenCourseWare