Table of Contents (click to expand)
The polymers, divided into three groups – caramelans (C24H36O18), caramelens (C36H50O25), and caramelins (C125H188O80) – are responsible for the browning of sugar. On the other hand, volatile compounds like diacetyl, Hydroxymethylfurfural, diacetyl etc. are responsible for the characteristic ‘caramel smell’.
When granulated sugar is first heated, it begins to melt. As it melts, its color starts to change from white to golden brown, and then to dark brown (if you continue heating it).

If you apply heat for even longer, it becomes black and gives off unpleasant fumes. It even tastes different at that point, but do you know what processes are at work here?
In order to understand why sugar turns brown when melted, and ultimately turns black, it’s imperative to first understand what sugar actually is.
Recommended Video for you:
What Is Sugar?
The term ‘sugar’ can be used for many chemical compounds – namely organic compounds (carbohydrates) that are sweet in taste and usually have a cyclic structure. The white stuff that we call ‘sugar’ is actually sucrose. It’s composed of 22 atoms of hydrogen, 12 atoms of carbon and 11 atoms of oxygen.
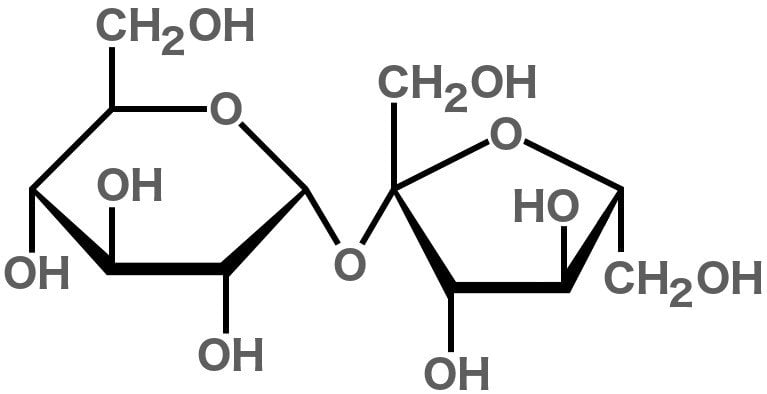
Just like all other compounds made from these elements (including fructose, glucose and maltose), sucrose is a carbohydrate. In fact, sucrose is actually two simpler sugars joined together: glucose and fructose. Sugar’s sweetness is attributed to the OH groups they contain; these OH groups with a particular orientation interact with the taste receptor for sweetness on our tongues.
Now that we know the basics of sugar, let’s proceed to the original question: why does sugar become brown when melted?
This is due to a process called caramelization.
What Is Caramelization?
Caramelization refers to the browning of sugar. It’s the process that causes sugar to acquire a brownish hue when it’s heated. You could also say that it’s the oxidation of sugar, or the degradation of sugars under heat.

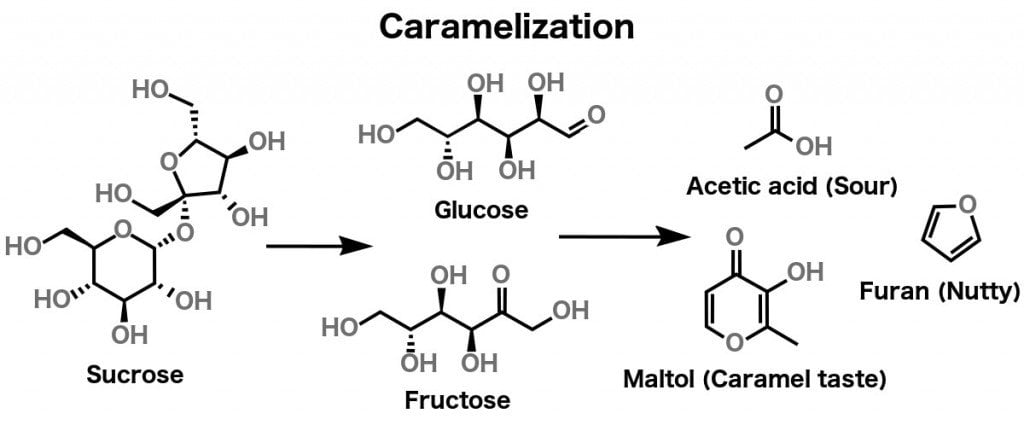
As mentioned earlier, different kinds of sugar (i.e., sucrose, fructose, glucose etc.) are carbon-based molecules. Caramelization happens to pure sugar when it is heated to 338 degrees Fahrenheit (170 Degree Celsius). As sugar reaches this temperature, it is broken down into simpler sugars, which then dehydrate and fragment into ketones and aldehydes. These polymerize and undergo various other reactions, but all of that is beyond the scope of this article.
What’s really relevant to our current discussion is that the compounds formed as a result of heating sugar can be classified into two categories: polymers and volatile compounds.
The polymers, divided into three groups – caramelans (C24H36O18), caramelens (C36H50O25), and caramelins (C125H188O80) – are responsible for the browning of sugar. On the other hand, volatile compounds like diacetyl, Hydroxymethylfurfural, diacetyl etc. are responsible for the characteristic ‘caramel smell’.
Interestingly though, caramelization is not the only process that causes browning of a sugary substance; a Maillard reaction (browning of meat, bread etc.) also does the same thing.
Caramelization Vs Maillard Reaction
It should be noted that both caramelization and Maillard reactions cause the browning of sugars under high heat, and in fact, caramelization may sometimes cause browning in the same foods in which the Maillard reaction occurs, but the two processes are NOT the same.
The primary difference between both the processes is that caramelization occurs when only sugar is heated to a high temperature, whereas the Maillard reaction occurs when both amino acids (from proteins) and carbohydrates (sugar) are present.

A lot of delicious everyday food items, including cookies, biscuits, breads, toasted marshmallows, seared steaks and others get their distinctive brown color and characteristic nutty taste due to the Maillard reaction.
The thing is that both caramelization and the Maillard reaction involve heat, cause browning, have similar effects on the taste buds and even work on similar food bases, so it’s easy to confuse the two processes.
You may have noticed that if you continue heating sugar after it has turned dark brown, it gradually turns black, and acquires a bitter ‘charred’ taste. That’s because the carbon, oxygen and hydrogen atoms in the sugar vaporize as different organic fragments, leaving behind a black, bitter version of sugar.













