Table of Contents (click to expand)
In single replacement reactions, one element replaces another in a chemical compound. In double replacement reactions, the swapping of elements takes place.
Imagine a hypothetical situation where a kid who loves ice cream is going out with his two friends. Initially, he is holding hands with Friend A, but Friend B soon joins then and offers his ice cream to the child. Whose hand do you think he will want to hold then? Obviously, Friend B! Kids are like that. They easily drift towards people who offer them the things they love. Funnily, chemistry also deals with these types of interactions, which are called reactions.

A replacement reaction is a type of chemical reaction in which one element replaces another in a compound. This can either be in the form of a single replacement reaction or a double replacement reaction. In a single replacement reaction, one of the reactants is more reactive than the other, which results in the formation of a product that is more stable. In double replacement reactions, the elements get replaced in both the reacting compounds. Double replacement reactions also result in the formation of a solid product, which is called a precipitate.
But how do these reactions take place? Let’s take a look!
Recommended Video for you:
What Is A Replacement Reaction?
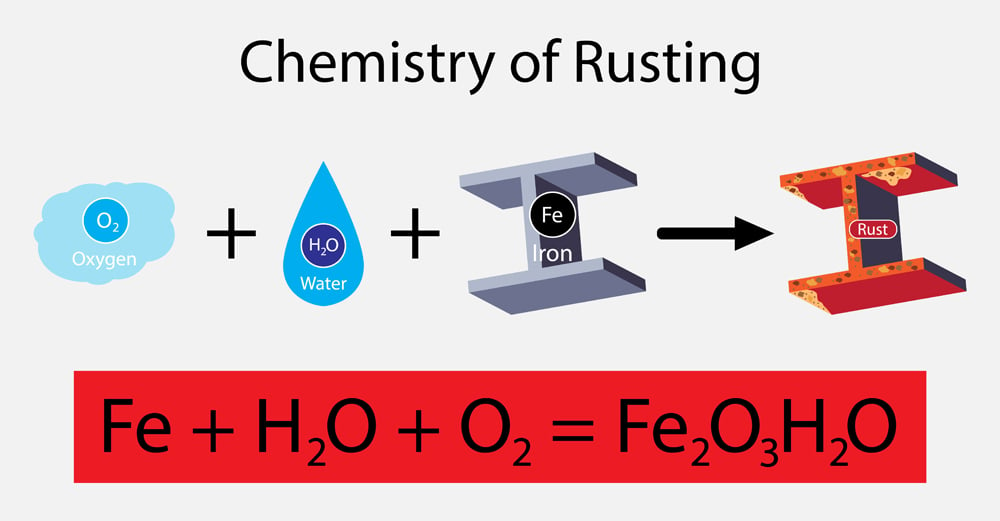
Before diving into the subtypes, let’s explore the basics of this chemistry principle. Have you ever seen a rusted iron rod? This type of object is a prime example of a chemical reaction. A process in which one or more substances are converted into one or more different substances is called a chemical reaction. The substances that undergo a change are referred to as reactants, while those being formed are called products. In the case of rusting, our reactants are represented by iron and water vapor in the air. When these two react, they form rust, which is referred to as our product.
However, to classify reactions and make things a bit easier, we have broken this down into specific categories. In replacement reactions, more reactive elements replace the less reactive elements of a compound to form a stable product. These replacement reactions are also known as displacement reactions.
What Are Single Replacement Reactions?
Now that you know what replacement reactions are, let’s take a look at one of its subtypes. Consider the following reaction: A + BC —— B + AC
Here, A is an element, and BC is a compound, but A is more reactive than the compound BC. Therefore, when they undergo a chemical reaction, A will replace B, as it is more reactive, to form the compound AC. B is given out in either an elemental or ionic form. Only a single compound undergoes replacement in this reaction, thus the name “single replacement”.
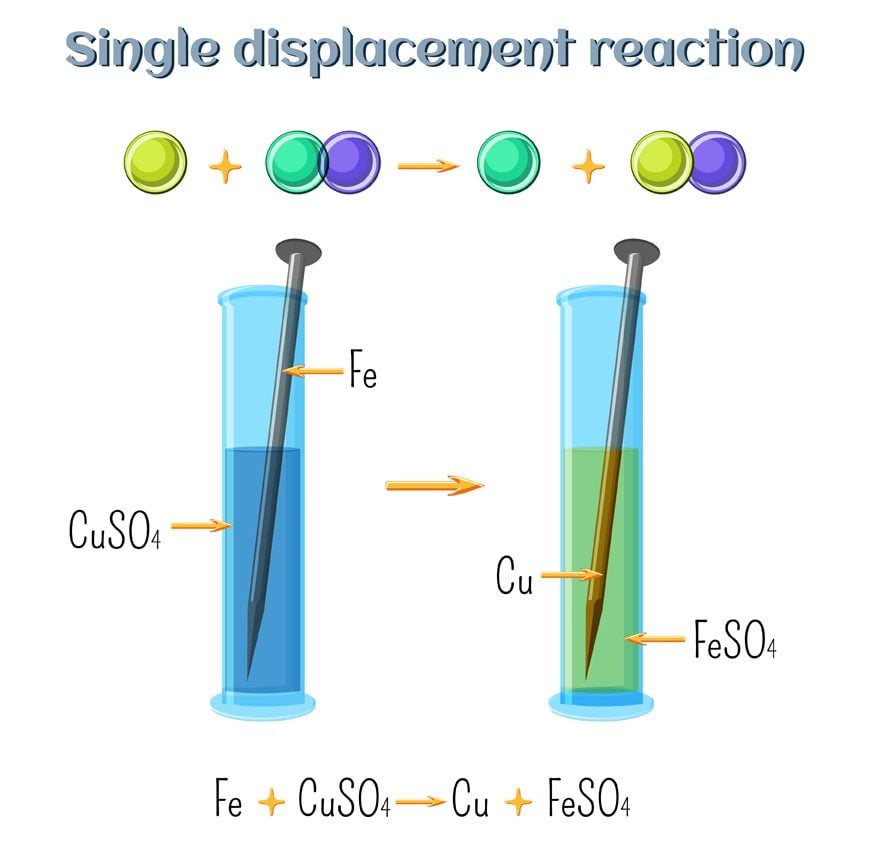
To understand this better, let’s consider the following examples:
Zn + CuCl2 —— ZnCl2 + Cu
This reaction easily fits in with the above explanation. Zinc is more reactive than Copper. Thus, when it is added in a solution of Copper Chloride, it replaces Copper and forms Zinc Chloride. Copper is given out in the ionic form. This reaction is also an excellent example of cationic replacement. We say this because the replacing ion has a positive charge, i.e., 2+. As such, it is a cation.
Br2 + 2KI —— 2KBr + I2
Similarly, when Bromine is added to a solution of Potassium Iodide, it replaces the position of Iodine in the compound. As a result, Potassium Bromide is formed, whereas a molecule of Iodine is given out. This reaction takes place because Bromine is more reactive than Iodine, which is why it replaces it. Because the replacement ions have a negative charge, this reaction is an example of anionic replacement.
What Are Double Replacement Reactions?
The easiest way to understand a double replacement reaction is by remembering the term “swapping partners”. In double replacement, the elements get replaced in both the reacting compounds. Here, parts of two ionic compounds are exchanged, making two new compounds. Let’s consider the following reaction: AB + CD —— AC + BD
Here, the elements in the reacting compounds exchange their partners. In the product, A combines with C, while B combines with D. Because the replacement occurs in two places, it is referred to as a double replacement. Double replacement reactions are also called metathesis or double displacement reactions. Usually, in these reactions, when combining aqueous solutions, a solid product is also formed. This product is referred to as a precipitate, and the reaction is called a precipitation reaction.
For example, take a look at this reaction:
AgNO3 (aq) + NaCl (aq) —— AgCl (s) + NaNO3 (aq)
When an aqueous solution of Silver Nitrate is mixed with an aqueous solution of Sodium Chloride, it results in the formation of a Sodium Nitrate solution, while Silver Chloride precipitates out. One can easily see that elements have been replaced in both the reacting compounds.
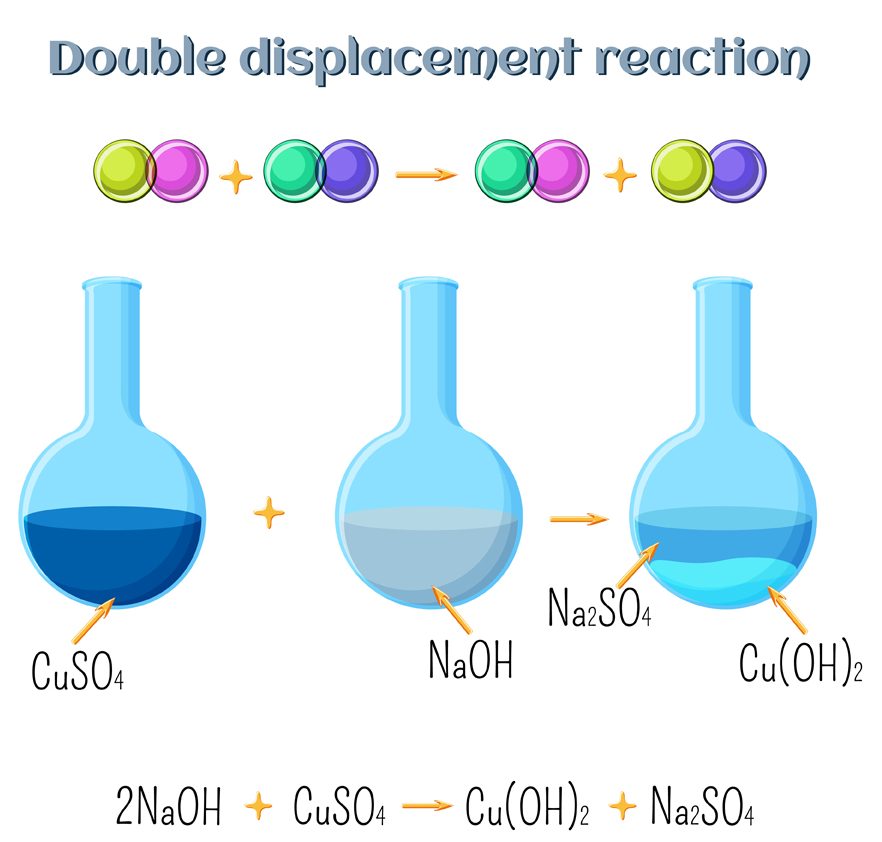
To summarize, in single replacement reactions, the more reactive element takes the place of a less reactive compound. On the other hand, in double replacement reactions, both of the reacting compounds swap partners and end up forming entirely new compounds!