Table of Contents (click to expand)
Water boils when the energy from the heat is great enough to break the hydrogen bonds between the water molecules. The gas that is released from the boiling water is made up of oxygen and carbon dioxide.
If you’ve ever boiled water, you will have noticed that as water heats up, very tiny bubbles are formed that rise from the bottom to the top. Initially, the bubbles are few and far between, but as the water becomes hotter, more bubbles of larger sizes start to form. Increasing the heat further results in even larger bubbles that form quite frequently and rise immediately to the top. This escalation reaches a peak when water starts to boil.
But why does boiling water make bubbles?
The answer to that has to do with the chemistry of water itself. More specifically, it has to do with all the dissolved substances in water, as well as the nature of bonding between water molecules.
Recommended Video for you:
Chemical Properties Of Water Molecules
Each molecule of water is composed of two hydrogen (H) atoms and one oxygen (O) atom. Both the H atoms are covalently bonded to the sole O atom. Every element in nature strives to reach a state of the lowest possible energy. This state is achieved by losing or gaining electrons to reach the nearest noble gas configuration.
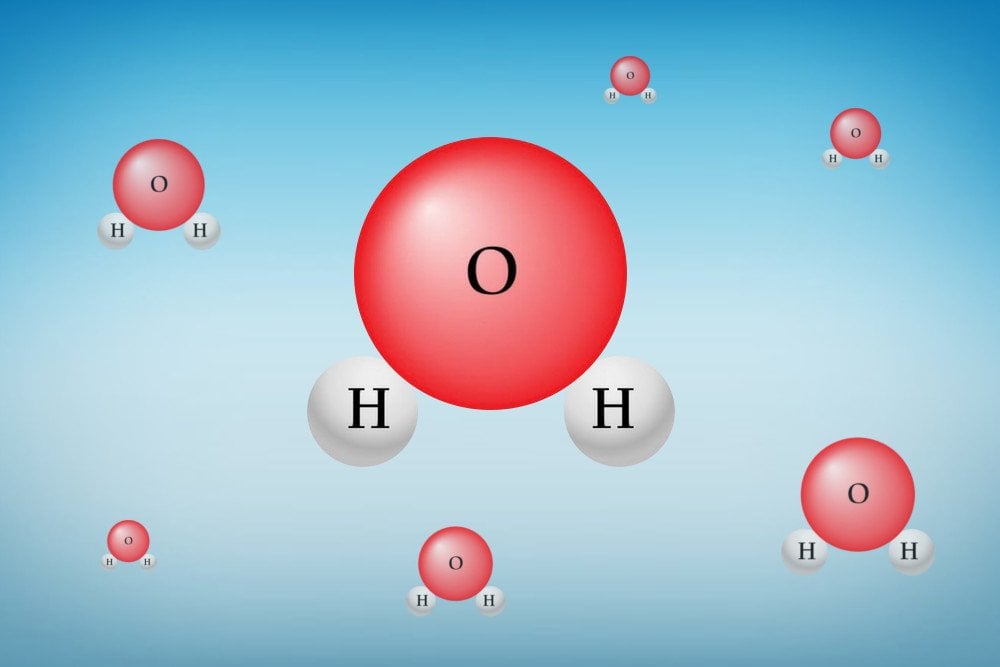
An oxygen atom has six electrons in its valence (outermost) shell. The nearest noble gas, Neon, has eight electrons in its valence shell. Thus, O has a strong tendency to gain two electrons and attain a stable electronic configuration (enter the lowest energy state). A hydrogen has one electron in its valence shell, while the nearest noble gas, Helium, has two electrons on its valence shell. Thus, H tends gain one electron to attain a stable electronic configuration.
Both the H atoms share an electron each with O, while O shares two electrons, one for each H. This is a covalent bond. Oxygen has a strong tendency to attract shared electrons towards itself, due to a property called electronegativity. Thus, electrons spend more time near the O atom than the H atom, resulting in a partial negative charge on O and a partial positive charge on H.
The geometry of a water molecule is such that the charges don’t cancel out, and there is a separation of charge centers (polarization). When two water molecules with slight polarization approach each other, the partially negative O of one molecule attracts the partially positive H of the other molecule to form a weak intermolecular bond. This is called a Hydrogen Bond, and is the force responsible for holding water molecules together.
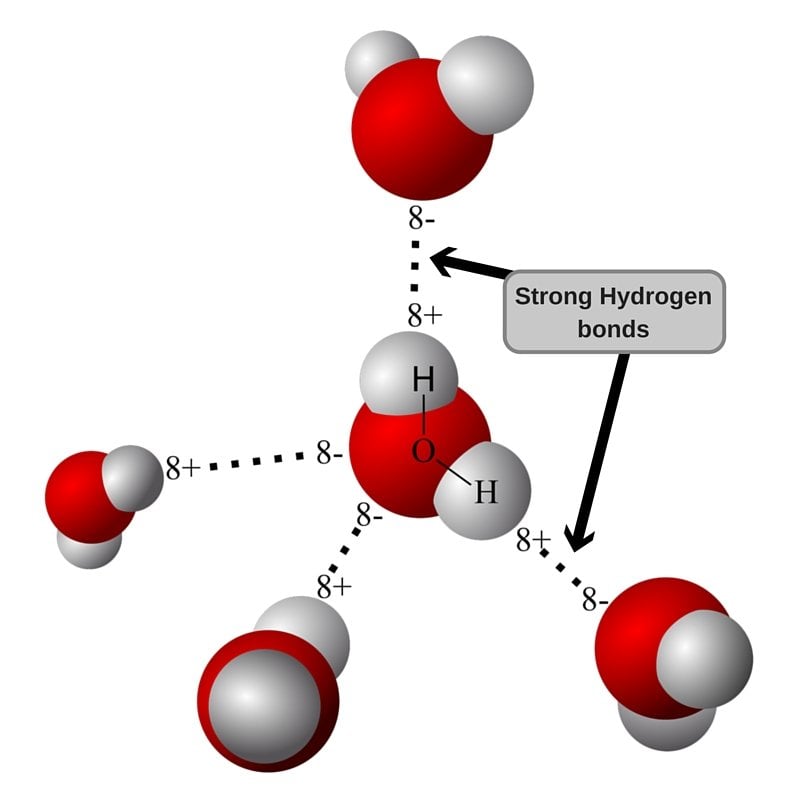
As the hydrogen bond is weak, water remains liquid at room temperature and as the temperature rises, the molecules gain more energy to overcome intermolecular hydrogen bonding. At 100oC, the energy is sufficient for the molecules to break free.
Dissolved Substances In Water
The dissolution of one substance in another is possible only when there is an interaction between the molecules of the two substances. Likewise, some gases, e.g., O2, CO2, N2, NH3, and SO2, get dissolved in water because there exists some attractive interaction between the water molecules and the gas molecules.
There are two ways that gases can dissolve in water: van der Waals bonding and Hydrogen Bonding.
Heteronuclear molecules (i.e., having atoms from different elements), like NH3 or CO2, have an electronegativity difference between the atoms. N and O are more electronegative than H and C, respectively. Thus, N and O remain partially negative and H and C become partially positive. This leads to the partial polarization of NH3 and CO2 molecules.
The negative ends (N and O) are attracted to to the partially positive H of water; meanwhile, the positive ends (H and C) are attracted to the partially negative O of water. This is hydrogen bonding. The greater the polarization of the gaseous molecule, the better it dissolves in water.
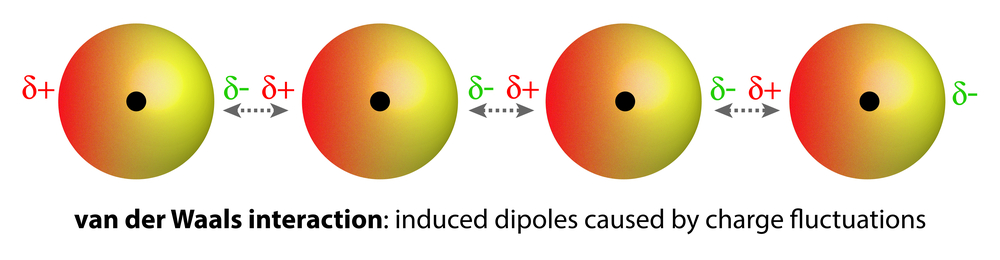
Homonuclear molecules (i.e., having atoms of the same element), like O2 and N2, are non-polar and are sparingly soluble (very low solubility) in water. Weak van der Waals forces of attraction hold these gases with water molecules. These are much weaker than dipole-dipole interactions.
The solubility of gases in water decreases as the temperature increases.
The Sequence Of Events When Water Boils
Let’s take liquid water at room temperature (25oC). At this temperature, the solubility of O2 is 8.27 mg/L and that of CO2 is 1.5 g/L. As the temperature increases, molecules of gas and water gain more kinetic energy. This energy makes it easier for all the molecules to overcome the intermolecular attraction. At 50oC, the solubility of O2 decreases to 2.75 mg/L and that of CO2 to 0.75 g/L. This decrease in solubility means that the gaseous molecules can overcome the weak intermolecular attractions. Since gas molecules have densities lower than that of water, they rise to the top as bubbles. Homonuclear molecules like N2 and O2 bubble out at lower temperatures because of the weak van der Waals forces. Increasing the temperature further results in the bubbling out of polar molecules like CO2 and NH3, which are held by dipole-dipole interactions.

This bubbling continues until the boiling point of water is reached. The heating of water is not completely uniform, meaning that there are regions of higher and lower temperatures. At temperatures above 90oC, some water molecules near the bottom gain enough energy to transition to the vapor phase. Regions of gaseous water are formed, which are indicated by huge bubbles rising up from the bottom. Also, due to the vigorous motion of molecules, convective heating raises the temperature further. At 100oC, almost all the water molecules have sufficient kinetic energy to transition to the vapor phase and bubbles of water vapor will begin to rise up rapidly!
References (click to expand)
- Limnology: 4.1. Dissolved gases – Oxygen, Carbon dioxide .... ecoursesonline.iasri.res.in
- Solubility. Florida State University
- Oxygen Solubility. Colby College
- Electronegativity - UW-Madison Chemistry. The University of Wisconsin–Madison
- Maximum Dissolved Oxygen Concentration Saturation Table. lakestewardsofmaine.org
- Solubility of Gases in Water vs. Temperature. engineeringtoolbox.com