Combustion is a redox reaction between fuel and an oxidant. Depending on the level of oxidation, the flame color in carbon fuels will also differ.
You have probably noticed that fires come in various sizes and colors. A burning candle wick gives off an orange-yellow flame, while a gas stove usually puts out blue flames. Other elements give an even greater variety of colors. For example, burning a piece of magnesium gives off a bright white flame, while potassium generates a purple flame upon burning.

Combustion, or burning, is scientifically explained as “a high-temperature exothermic redox reaction between the fuel and the oxidant”. It is a reaction in which high amounts of heat are produced, thus making it exothermic. A redox reaction is where one substance gets reduced (loses oxygen) while the other gets oxidized (gains oxygen). The molecule that provides oxygen to the other is the oxidant. Here, the fuel is getting oxidized, while the oxidant is losing its oxygen.
Fire, or flames, are the physical effects of combustion that are visible to the naked eye (most of the time). So what causes flames of different colors? In fact, why does combustion produce visible flames at all?
Recommended Video for you:
Why Are Flames Produced?
Combustion is an exothermic reaction. The word “exothermic” is made of two parts: “exo” meaning releasing, and “thermic” meaning heat. These types of reactions produce a significant amount of heat when they take place.
These reactions produce heat because a huge amount of energy is released as the molecules undergo chemical changes. However, heat is not the only form of energy. These reactions also release energy in other ways, including the release of energy through photons, which produces light!
The release of photons requires a lot of energy. It is often also accompanied by a good deal of heat, especially during combustion. When atoms release energy, their electrons drop to a lower energy state, which is how they emit light. To understand this better, one must know some basics about atoms and their orbitals.
Atoms And Their Orbitals
Atoms are the basic building blocks of all matter in the universe. They are the smallest functional unit that forms all organic and inorganic matter. While an atom can be broken down further, its constituents cannot exist individually. They are not physically stable on their own, so an atom is the smallest stable entity that can exist on its own.
The constituents of atoms are as follows—neutrons, protons and electrons. The neutrons and protons are bound together and form the center, or the nucleus, of the atom. They also constitute the entire mass of the atom. Electrons, on the other hand, are extremely small in size and have negligible mass in comparison to the nucleus components.
Electrons revolve around the nucleus of the atom, much like planets revolving around the sun in our solar system. Just like the solar system, the atom also has paths on which the electrons revolve around the protons and neutrons. These paths are known as orbitals.
Every orbital in an atom has some potential energy. Therefore, depending on which orbital it is occupying, an electron will have different energy levels. As you go farther from the nucleus, the energy level of the orbital increases. Electrons in these orbitals will have higher amounts of energy.
Excitation And De-excitation Of Electrons
Electrons in different orbitals have different energy levels. The level of potential energy that an electron has is its energy state. Electrons in higher energy orbitals have higher energy states.
It is also possible for electrons to jump from one orbital to another orbital within the atom. Due to differences in energy levels, the electrons must also compensate for this energy change.
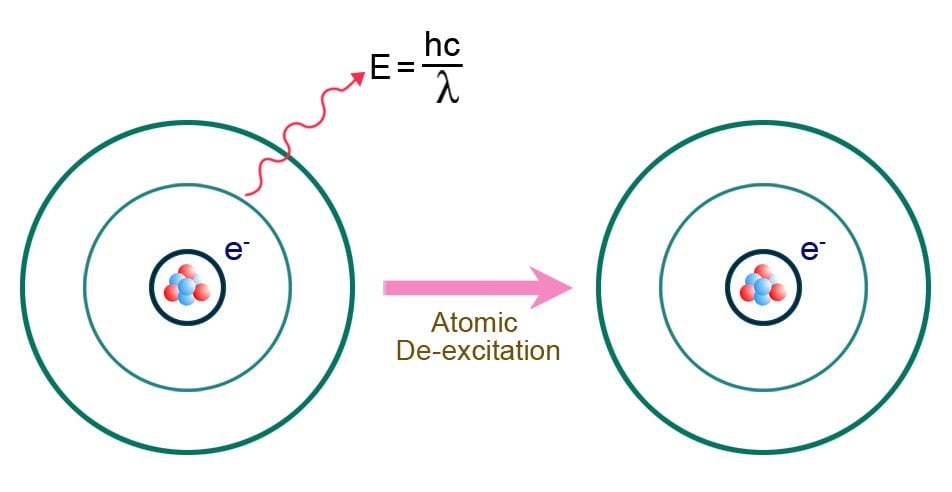
When going from a lower energy state to a higher one, an electron must absorb energy from its surroundings. This is known as the excitation of the electron. When going from a higher energy state to a lower energy state, the electron releases energy into its immediate surroundings. This is called the de-excitation of the electron.
This kind of energy change is central to most chemical reactions. All molecules try to achieve the lowest possible energy state in order to gain stability. During combustion, the atoms lose a lot of energy, so the de-excitation of electrons occurs.
How Does The De-excitation Of Electrons Produce Different Colors Of Light?
The de-excitation of electrons, as mentioned above, releases energy into their immediate surroundings. One of the most common ways to release energy is through the emission of light. When an electron loses energy, it transfers that energy to a photon.
Photons are the fundamental particles of light. They are subatomic particles that are massless and carry energy in the form of light. Essentially, all light is made of photons. The wavelength of the emitted light depends on the energy absorbed by the proton.
When the energy from an electron transfers to a photon, light is emitted from the atom. Depending on the energy change of the electron, the energy provided to the photon will also vary. This results in different wavelengths of light being emitted by different energy changes.
Since varied wavelengths correspond to their own individual colors, it results in the emission of different colored lights. Depending on what molecule is undergoing combustion, the electron energy state changes. Thus, the combustion of different substances produces different colored lights.
Levels Of Oxidation Of Carbon Affect The Flame Color
The most common fuels that we use in everyday life are carbon-based. From LPG cylinders to candle wax, most of the combustion around us involves carbon-based fuels.
In that case, why does a candle flame differ from a gas stove? Because of the level of oxidation the carbon molecule goes through.
In the presence of enough oxygen, all the carbon atoms turn to carbon dioxide, and the flame appears blue in color. However, in the absence of enough oxygen, not all carbon forms carbon dioxide. Carbon monoxide and particulate carbon are also produced. The particulate carbon forms soot.

You must have noticed the presence of soot in the form of blackish powder. Soot is formed when there is not enough oxygen available to oxidize all the carbon that is present. The wavelength of light emitted by carbon particles when it is not completely oxidized corresponds to a yellow color.
On the other hand, when carbon is in its completely oxidized form, i.e., carbon dioxide, the de-excitation of electrons has a different energy. In this case, the wavelength of light produced corresponds to a blue color.
In the case of complex carbon molecules like wax, paper and wood, the ratio of carbon to atmospheric oxygen is very high. The oxygen in the air is not enough to oxidize all the carbon atoms. This gives rise to the orange-yellow flame seen in these cases. However, atmospheric oxygen is enough to completely oxidize the carbon in simpler fuels like methane and LPG, which gives them a characteristic blue flame when they burn.

Conclusion
Combustion is the burning of molecules to produce heat and light. Different molecules produce different colored flames. The color of each flame depends on the energy released by the electrons of the atom during de-excitation.
In the case of carbon fuels, the color of the flame depends on the amount of oxidation that the carbon molecules undergo. In the presence of sufficient oxygen, all the bonds are broken, and all the carbon is released in the form of carbon dioxide. This gives off a blue flame. In the case of insufficient oxygen, not all the carbon is turned to carbon dioxide. Instead, soot forms as well. Soot is composed of pure carbon molecules. Particulate carbon matter burns with a bright yellow-orange flame.
The complete combustion of carbon-based fuels produces blue flames. While atmospheric oxygen is enough for the burning of simple carbon fuels, like methane and LPG, it is not enough for more complex substances. Hence, organic substances like wood and wax burn with a yellow flame. This is why some flames appear blue, while others appear yellow!