A dipole is a substance that incorporates two equal and opposite charges (possessing a charge can be thought of as possessing some amount of energy). The charge can be electric, magnetic or any other type.
Everything has two sides. This is the law of nature that has been followed since the beginning of time. As a matter of fact, the birth of our universe was brought about by the division of nothingness into matter and anti-matter. These two opposite forms of energy are the basis of every substance that exists under the laws of our world. Our planet, being one of these entities, also follows this law of opposites.
Why is this so? Well, the existence of these opposing sides begins at an incredibly small scale—the molecular level. All the atoms and molecules that constitute matter are dipolar.
Recommended Video for you:
What Is A Dipole?
A few weeks ago, two of my very good friends were having a healthy debate about the true meaning of the word dipole. Sam began his side of the argument by pointing out that the word dipole is composed of two smaller words- ‘di’, which means ‘two’, and ‘pole’, which means the ‘end or extremity of something’. Bill joined the discussion by quoting Merriam-Webster, which defines dipole as a pair of equal and opposite electric charges that are separated by a very small distance.
However, Sam pointed out that the two ends of a magnet are often referred to as magnetic dipoles, so the word dipole actually represent magnetic substances. So… who is correct?
To be fair, neither of them is wrong, per se. A dipole is a substance that incorporates two equal and opposite charges (possessing a charge can be thought of as possessing some amount of energy). The charge can be electric, magnetic or any other type. In this context, we can differentiate between an electric dipole and a magnetic one.
What Is The Difference Between An Electric And A Magnetic Dipole?
An Electric Dipole is formed from a substance that possesses two equal electric charges of opposite sign separated by a small distance. The best example of an electric dipole is an atom with one electron and one proton (Hydrogen). The proton acts as the source of positive charge, while the electron possesses an equal amount of negative charge.
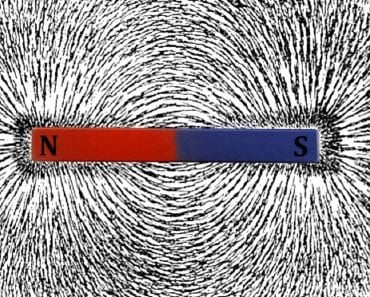
A magnetic dipole is quite similar to an electric dipole, except that it’s composed of magnetic charges. A simple bar magnet is the best example of a magnetic dipole. One end of the bar constitutes the north pole, while the other end acts as the south pole. The amount of charge at both ends of a uniform bar would be identical. The north pole attracts the south and vice versa.

What Is A Dipole Moment?
A dipole moment is a measurable quantity, just like mass, volume or speed. It is the characteristic by which dipolar substances can be quantified and differentiated.
Dipole Moment Formula
The formula to calculate the dipole moment of a simple system with two charges states that the dipole moment is the product of the magnitude of the charge and the distance separating the opposite charges. More complex systems have more complex formulas, all of which are derived from the basic one.
For example, consider a system consisting of two opposite electric charges of 1 coulomb each, separated by a distance of 1 nanometer. The dipole moment of such a system would be 10−9 coulomb-meter.
Coulomb-meter is an SI unit that applies for the electric dipole moment. However, molecular dipole moments are too small to be effectively measured by this unit.
Therefore, for measuring dipole moments of molecular bonds, a unit called Debye (D) is used (1D=3.33564×10−30 C·m).
On an atomic level, dipoles occur due to non-uniform charge distribution. Consider an atom with a sea of electrons revolving around the central protons in fixed orbits but random order. Now, pause the scenario at any instant of time and select a pair of proton and an electron.
What do you see? A dipole is formed by the pair of equally and oppositely charged species. Similarly, other dipoles, equal to the number of electrons in the atom will be formed and their dipole moments will combine and result in a net dipole moment of the species.
What Is The Difference Between A Permanent And A Temporary Dipole?
Permanent dipoles arise due to the difference in the electronegativity (relative attraction towards electrons) between two atoms inside a molecule.
Let’s consider hydrogen fluoride as an example. The electronegativity of fluorine is greater than hydrogen, and as a result, fluorine pulls all the electrons towards itself and becomes the negative pole. Hydrogen, in turn, has an excess of protons and becomes the positive pole. Such molecules with permanent dipole moments are called polar molecules.
Instantaneous dipoles are temporary dipoles. Inside an atom, electrons revolve around the nucleus in fixed paths, but their motion is random. Sometimes, these electrons become more concentrated in one particular region than in another. This gives rise to an instantaneous or temporary dipole. The mechanism is similar to what was explained in the formation of atomic dipoles.
These dipoles are small in magnitude and are only relevant for a very short period of time. However, they play a major role in many molecular phenomena. The London dispersion forces seen in several non-polar molecules such as oxygen and carbon-dioxide are its best examples.
What Is A Quadrupole?
As researchers around the world discovered more and more about dipoles, they were faced with one curious ambiguity—is a dipole the limit? Their questions were soon answered with the discovery of a quadrupole.
A quadrupole consists of four different monopoles of opposite signs (two of each) and of equal magnitude arranged in a perfectly symmetric distribution to represent an ideal system. The monopoles can be an electric charge, magnetic poles or even a simple mass. As already mentioned, mass is a polar entity. The opposite of mass or matter is called anti-matter, and you can read more about antimatter in this article.
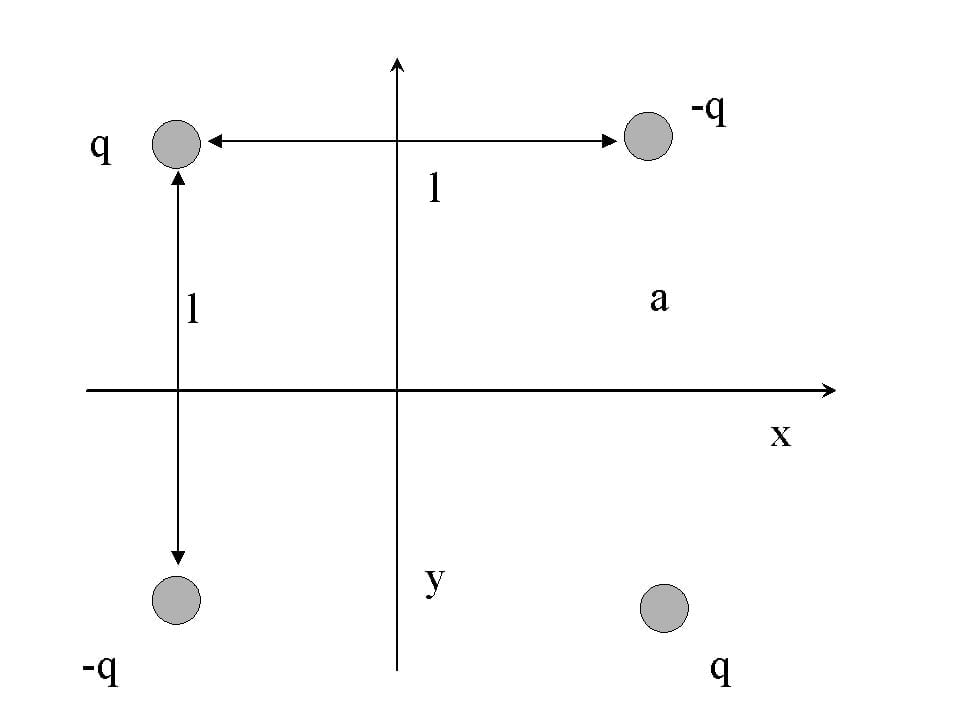
To understand quadrupoles better, let’s look at an example.
Consider two positive electric charges and two negative ones. Arrange these charges on the corners of a square in an alternating fashion. The end result would look something like what is shown above in the figure. The total net charge of this system would be zero, as would its dipole moment since the two opposite dipoles cancel each other out.
However, the quadrupole moment of this system can never be reduced to zero, regardless of the orientation of the particles. The exact reason for this phenomenon is still being researched around the world!
Conclusion
From what we learned about dipoles so far, one thing is pretty clear, they are very simple in principle and equally complex in working. A lot of phenomena that happens around us can be attributed to the dipolar nature of atoms and molecules. These include simple things like the fluidity of water as well as complex stuff like the relaxation of biological tissues and other things that are out of the scope of this article.
In conclusion, though their function might not be noticeable in daily life, dipoles are very important for us.