Table of Contents (click to expand)
By definition, osmosis is the movement of any solvent through a selectively permeable membrane into an area of higher solute concentration, the result of which will be an equalizing of solute concentration on either side of the membrane.
There are many times in life where you have heard someone preach about the importance of balance. As a general concept, keeping things balanced is widely promoted as the best way to increase your health, happiness and attitude.
It may seem like an abstract concept, but it is present in so many parts of our daily life, ranging from the balance of foods we eat and the time we spend at the office vs. home, to the political beliefs we hold and our ability to physically stand up straight while walking down the street.
Like it or not, finding balance is an important element of survival.

As is so often found in this beautiful universe of ours, the microcosm reflects the macrocosm, and balance is just as important on the cellular level as it is in our daily lives.
When it comes to the microscopic level, however, finding balance has a great deal to do with solutes and solvents, concentrations of materials inside and outside of cells, which determine the amount of water that needs to move into or out of the cells. Achieving this proper, healthy balance requires a passive, perpetual process, one on which all living cells depend – osmosis!
Recommended Video for you:
What Is Osmosis?
By definition, osmosis is the movement of any solvent through a selectively permeable membrane into an area of higher solute concentration, the result of which will be an equalizing of solute concentration on either side of the membrane.
This equilibrium is important for the efficient and optimized function of cells; as mentioned before, balance is the preferred state in a natural environment.
While any solvent can undergo the process of osmosis, including supercritical liquids and some gases, the majority of discussion surrounding osmosis relates to the movement of water in cells. The regulation of water movement throughout our entire body is done through the manipulation of solute concentrations and osmosis. The absorption or diffusion of water helps to unconsciously provide stability and functionality to every cell, tissue and organ in our body.
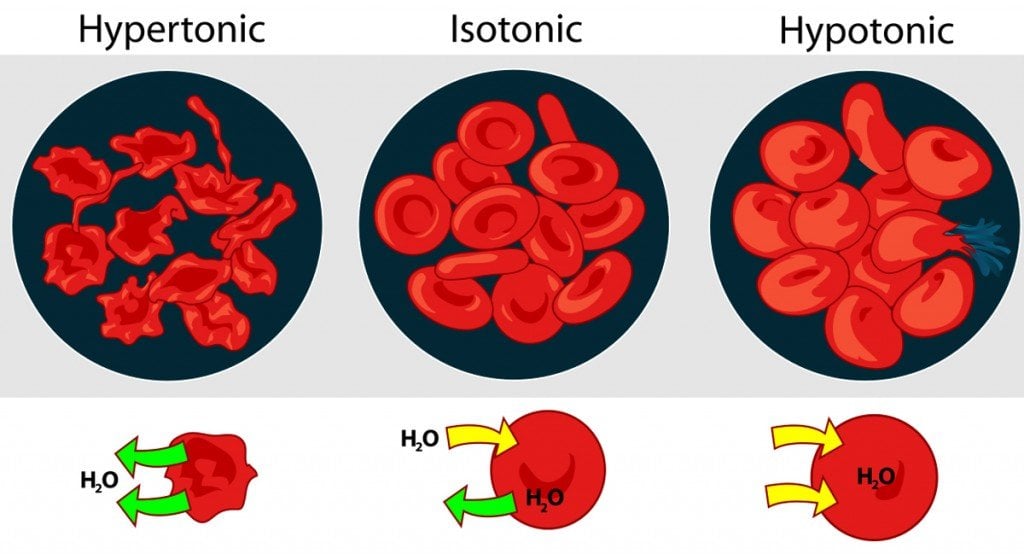
Three Types Of Osmotic Solutions
There are three types of solutions – isotonic, hypertonic and hypotonic solutions – in which osmosis plays a key role and occurs differently; understanding these basic examples is necessary before learning about the cool and more complex details of osmosis, as well as its importance on so many aspects of our survival.
Isotonic Solution
If you place a cell in an isotonic solution (in relation to the cell), the concentration of solutes is even, meaning that water can move into and out of the cell at an equal rate. Osmosis will occur, but at a balanced pace, meaning that the cell won’t swell or shrink. For example, if both your solvent and your cell’s cytoplasm are composed of 75% water and 25% salt, the concentrations are equal and there is no net movement of water across the selectively permeable membrane.
Hypotonic Solution
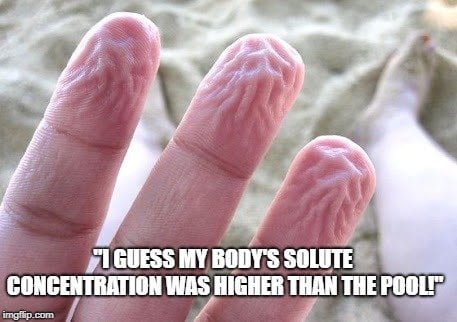
If you place a cell in a hypotonic solution (in relation to the cell), water will flow into the cell in order to equalize the solvent concentration. The best example of this situation is the way your fingers prune after you go swimming. The concentration of salts and other solvents in your skin cells is higher than the water of the lake or pool, so water moves into those cells, causing them to swell and wrinkle. Imagine placing a cell with 60% water and 40% salt into a solution with 80% water and 20% salt. Water would flow from the solution into the cell until a balance of 70% water and 30% salt was achieved. In extreme cases within a cell, when too much water is taken in, it can cause the cell to swell and lyse (burst), causing cell death.
Hypertonic Solution
When a cell is placed in a hypertonic solution (in a relation to the cell), there will be a higher concentration of solute outside of the cell, so water will diffuse away from the cell, towards the hypertonic solution, in order to balance the solvent concentrations. Essentially, the cell will be sucked dry, and made flaccid, to compensate for the excess solute outside the membrane. In extreme cases, the cell shrivels enough that the cell membrane detaches from the cell wall and becomes plasmolyzed. Without water to move the various molecules in the cell, it will die.
Practical Importance Of Osmosis
Now that you understand the basic processes of osmosis, and what different conditions will cause osmosis to occur, you will be able to see the value of this process in so many areas for every form of life.
For plants, osmosis is responsible for the movement of water into the root system, which allows the plant to grow and survive. The root hairs of plants are the key point where minerals and water are taken into the organism. The concentration of water molecules are less in the root hairs than in the soil (hypertonic solution), so water moves into the cells of the root hairs; osmosis continues through numerous layers of cells (cell-to-cell movement) until that water reaches the xylem tubes – equivalent to human veins.

On a related note, when water is taken into the cells of plants, the pressure caused by that osmotic movement is called turgidity. When equilibrium is achieved, those plant cells should be full of water, as well as firm and turgid. This prevents leaves from wilting, allowing them to increase their surface area for sunlight capture. Osmosis also helps protect plants against drought and frost damage, as well as in regulating the opening and closing of stomata.
For animals (humans), some of the key osmotic functions relate to the balance of water content in the blood versus the surrounding tissues. Similarly, in the kidneys, osmosis controls the amount of waste buildup by increasing fluid flow into that organ. When the solute concentration is higher in the kidney cells (hypertonic solution), water is pulled from the body’s bloodstream into the kidneys (nephrons), which will eventually stimulate the need to urinate in a person/animal, thus eliminating those unwanted waste products.
A Final Word
Wherever water is present in the body, which is essentially everywhere, osmosis will be happening. Maintaining the concentration balance of solvents and solutes is a full-time, full-body job. The full extent of osmotic behavior in the body is beyond the scope of this article, but suffice to say that without osmosis, life as we know it wouldn’t be anywhere close to possible!
References (click to expand)
- Osmosis.
- OSMOSIS.
- http://web.archive.org/web/20190911091733/http://faculty.southwest.tn.edu:80/rburkett/GB1-osmosis.htm
- http://web.archive.org/web/20220626034619/http://www.brooklyn.cuny.edu/bc/ahp/LAD/C5/C5_Osmosis.html
- Osmosis - rheneas.eng.buffalo.edu:80
- Hinwood B. G. (1997). A Textbook of Science for the Health Professions. Nelson Thornes