Table of Contents (click to expand)
Oxidation is the process when an atom loses an electron in a reaction with oxygen and water. A browned apple or a rusted bicycle are common places where oxidation can be seen.
Oxidation is a common phenomenon; a browned apple or a rusty bicycle are both common examples of oxidation reactions. Oxidation does not mean that an oxygen atom is added to the compound. Instead, it is a chemical reaction that involves the loss of electrons. Metals are generally considered to be elements that can easily lose electrons, so they are known to be easily oxidized.
This lost electron does not wander around in the universe aimlessly; it is taken up by another atom. When one atom accepts an electron, it is called a reduction reaction. Oxidation and reduction go hand-in-hand and are jointly referred to as redox reactions.
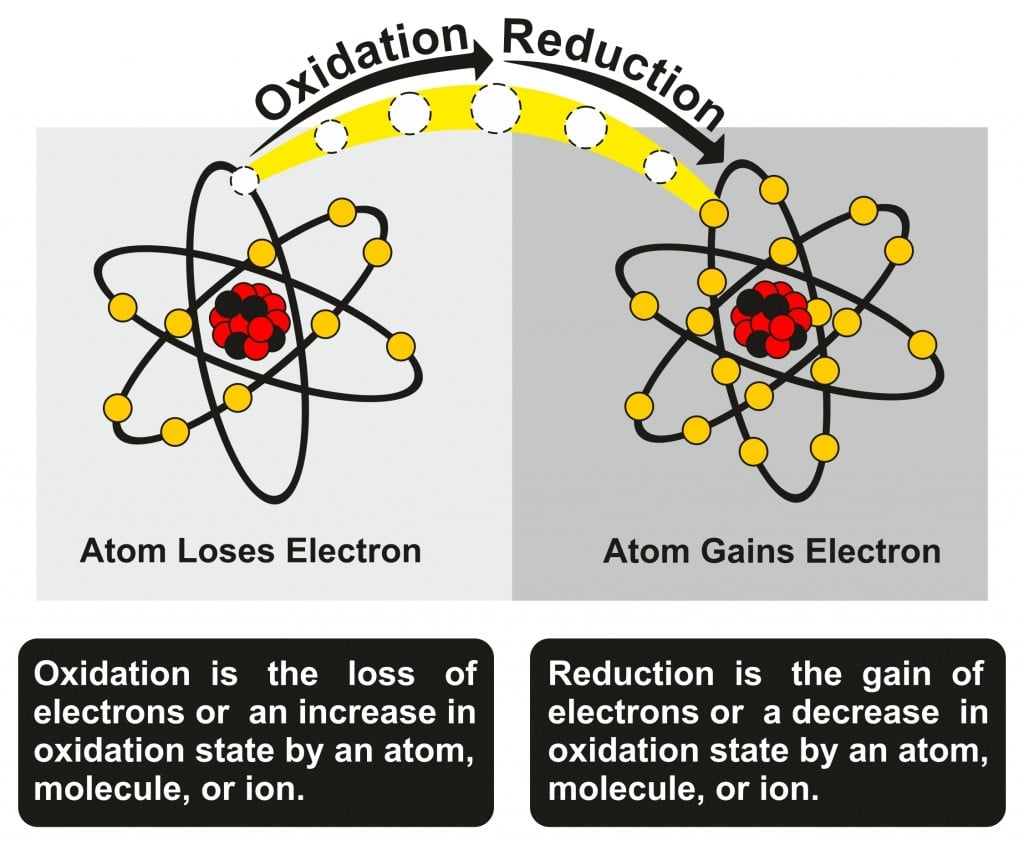
A simple way to remember oxidation and reduction is OIL-RIG; Oxidation Is the Loss of electrons and Reduction Is the Gaining of electrons. You can thank me later!
To make things easier, we’ll only be discussing oxidation reactions in this particular article.
Recommended Video for you:
What Is An Oxidation Number/state?
An atom is said to be oxidized when it loses an electron. The number of electrons that will be lost depends on the oxidation number of the atom or molecule.
The oxidation number or oxidation state is the charge of an atom. It can be positive, negative or zero. Oxidation numbers don’t always correspond to the real charges of the molecules.
An atom will have an oxidation number of zero when it is in its elemental state. This state of an atom is when it has ground zero-configuration or is in a neutral state. Sodium has 11 electrons; this is its ground zero state. In nature, the oxidation number is rarely zero, as atoms always tend to gain or lose electrons to form compounds.
The oxidation number depends on the atom’s electronegativity, which is predetermined by its position on the periodic table. As a rule, elements on the left side of the table are less electronegative and are therefore able to lose electrons easily. As a result, they have a positive oxidation number. Group I elements typically become oxidized, as they lose their valence electron while forming chemical bonds. Their oxidation number is +1. A valence electron is an electron in the outermost shell of an atom.
This property of elements changes as we move towards the right side of the table, where the elements tend to gain electrons and have a negative oxidation number. Take halogens, for example, which are extremely electronegative and usually undergo a reduction reaction. They gain one electron in order to have a configuration similar to that of noble gases. This leaves them with an oxidation state of -1. The oxidation number is negative, as they have gained 2 electrons, as opposed to losing electrons.
When it comes to a hydrogen atom, it only has one electron in its ground zero configuration and can either gain or lose an electron. This gives hydrogen a +1 or a -1 oxidation state. The hydrogen atom can be a reducing agent or an oxidizing agent, depending on the element with which it interacts.
What Is An Oxidizing Agent And A Reducing Agent?
An oxidizing agent is a substance that oxidizes other substances. This means that it allows other substance to undergo oxidation. To break it down even further, when an oxidizing agent is present in a reaction, it causes other atoms in the reaction to lose an electron. These lost electrons are accepted by the oxidizing agent. As a result, oxidizing agents are called electron acceptors.
This is like when you hire someone to do some work and take some of the load off your shouders. This outside agent takes the burden of work away from you, while becoming over-worked in the process.
In other words, a reducing agent is a substance that helps other substances get reduced in a reaction. The reducing agent helps the substance gain an electron.
An atom that gains an electron is called an oxidizing agent, as it allows the other atom to lose the electron and undergo oxidation. On the other hand, an atom that loses an electron is a reducing agent, as it allows the other atom to gain an electron and undergo reduction.
Think back to the abbreviation OIL-RIG; without this fundamental understanding, it will be difficult to fully comprehend the process.
How Does Iron Rust?
Rust, or iron oxide, is the most common example of oxidation. The shiny metal (iron) develops a brown layer upon reacting with oxygen and water. The reaction is quicker in seawater, as salt tends to corrode the metal rapidly. This is due to electrochemical reactions, where a transfer of electrons takes place between 2 substances—a solid and a liquid.
Rusting doesn’t occur in dry air, but instead occurs when air is accompanied with water. Humidity is an important factor for the rusting of iron.
The simple reaction is:
Iron + water + oxygen Hydrated iron oxide
More specifically, the reaction is as follows:
2Fe2+ + 4OH– → 2Fe(OH)2 (iron hydroxide)
The reaction involves an ion of iron and the hydroxide ion (hydrogen and oxygen), which reacts to give us iron hydroxide.
This iron hydroxide further reacts with oxygen to give rust, Fe2O3.
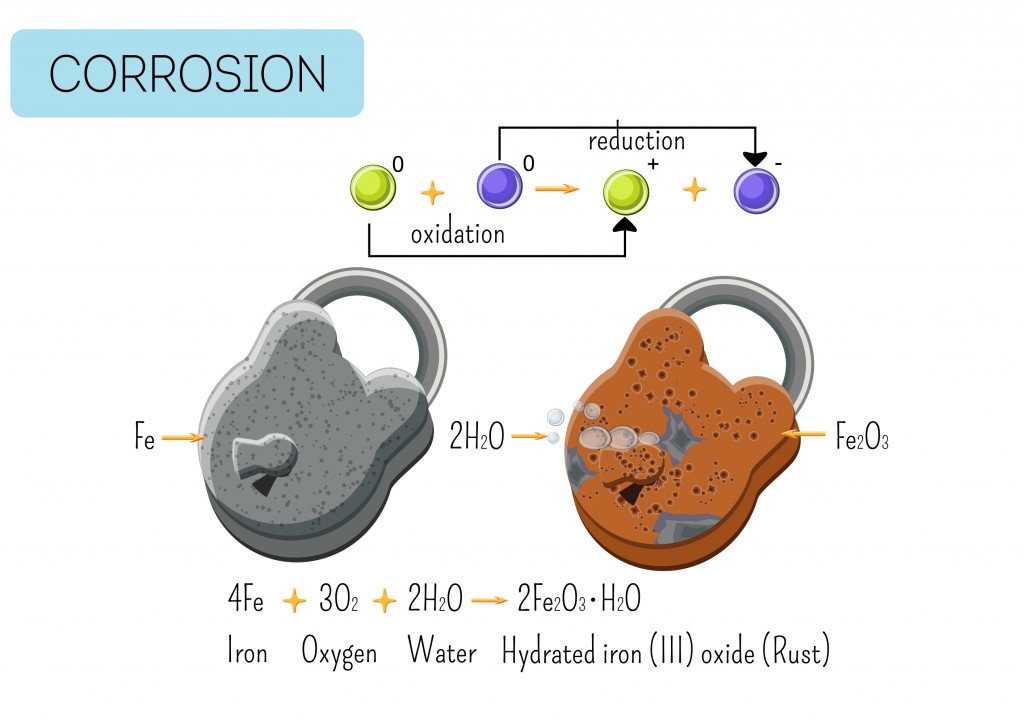
Both free oxygen gas (O2) and carbon dioxide (CO2) present in the air can be a source of oxygen for the formation of rust. Once rusting has settled in, the metal begins degrading further, eventually becoming brittle and unsuitable for use.
Conclusion
While it might be time to say goodbye to an old rusted bicycle, there’s still hope for other metallic items. The dial of your wristwatch, for example, is made of stainless steel and will rarely have the same fate as a bicycle. Stainless steel prevents the items from corrosion, as it separates the surface of the metal from air and water, allowing it to last for many years.
Stainless steel is an alloy, meaning that it is a combination of metals, along with various other elements. It consists of iron, chromium, manganese, silicon, carbon and in some cases, small amounts of nickel and molybdenum. These elements react with oxygen in the presence of water to form a thin and stable film. The film prevents corrosion by erecting a barrier to air and water.
Oxidation isn’t a bad thing, just an unwelcome guest who is difficult to eliminate; fortunately, taking proper care of metallic things can help prevent or slow oxidation. Applying lubricant or a protective coating might help, although this isn’t a foolproof method.
You can’t escape the effect of oxidative reactions that occur in nature; the only way out is to take preventive measures to delay their onset or mitigate their spread!