Table of Contents (click to expand)
Rancidity occurs when fats and oils are exposed to air, light, moisture, and bacteria, leading to hydrolysis or oxidation. This renders food items unfit for consumption.
Have you ever wondered why a bag of chips is always half full? The reason has to do with a concept called rancidity. No food item can remain fresh for an extended period. They become spoiled due to various chemical reactions, and rancidity is one of them.
When food items containing fats and oils are exposed to air or moisture, they become spoiled. To prevent chip spoilage, manufacturers flush nitrogen gas into the chip bags. Nitrogen is used in bags of chips because it does not react with fats and oils.
Recommended Video for you:
How A Substance Turns Rancid?
Rancidity describes the spoilage of food products containing oil and fatty acids. Fatty acids, present in fats, cholesterol, and steroids, are composed of carboxylic acids with a lengthy aliphatic chain. These acids can exist in either a saturated form, containing a single linkage between carbon atoms, or an unsaturated form, with multiple linkages between carbon atoms.
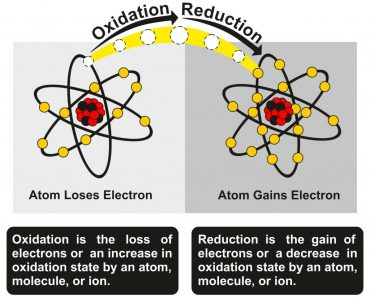
Rancidity Reactions Typically Occur In Three Steps:
1. Initiation reaction, which is stimulated by the action of external factors, such as heat and air, leads to forming radicals on the food substance. By definition, a radical is an atom, molecule, or ion that has an unpaired electron. This makes the “radical” more chemically reactive.
RH———>R*+H*
2. Propagation reaction, where oxygen gives rise to peroxides. These peroxides react with more unsaturated fatty acids and produce new radicals.
R*+O2———>ROO**
(peroxide)
3. Termination reaction, where two radicals combine and form a new single bond.
ROO*+ROO*———–>end products
At the end of rancidification, fats, oils, and other lipids are decomposed, thus forming highly reactive molecules. These are responsible for the unpleasant smell and taste of rancid foods. In some cases, rancidification may also lead to the loss of vitamins in food.
Types Of Rancidity
Rancidity can be classified into two major types: oxidative rancidity and hydrolytic rancidity.
Oxidative Rancidity
Oxidative rancidity is a reaction that damages a food substance by causing oxygen damage. This process interrupts and damages the natural oil structure in a way that can alter its color, odor, and taste. Furthermore, it forms toxic compounds like peroxides, which can destroy vitamins A and E in foods. The reaction also creates polymeric materials and oxidized sterols.
Hydrolytic Rancidity
Hydrolytic rancidity generally causes an unpleasant odor. This is due to the release of free fatty acids from glycerides.
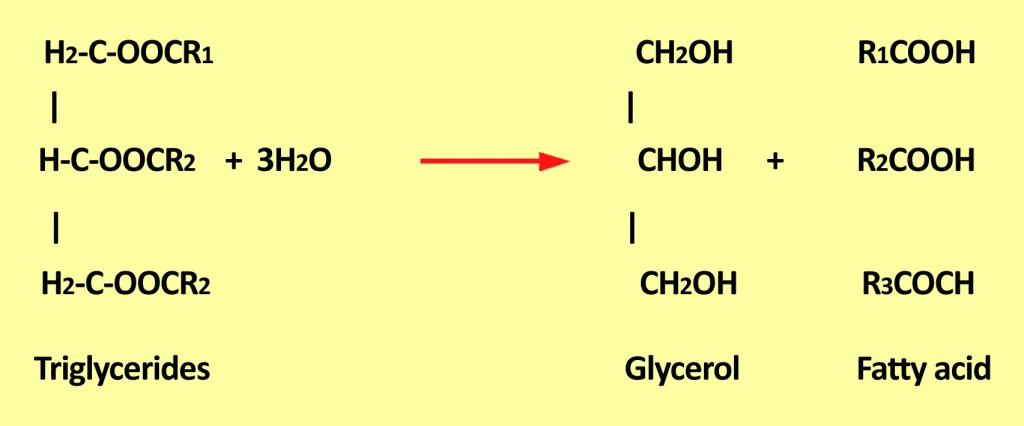
Triglycerides are a combination of three fatty acids that are generally present in oil foods. When they react with water, they produce glycerol and release free fatty acids unsuitable for consumption.
These fatty acids may further undergo oxidative rancidification, forming toxic compounds.
Factors That Affect Rancidity
- Oxygen: Exposure to oxygen is the primary cause of rancidity. Oxygen is more soluble in fats, which leads to oxidation and food damage by releasing free radicals.
- Microorganisms: Several microorganisms release an enzyme called lipase that leads to the hydrolysis of lipids. This enzyme further acts on oils and fats. Lipase requires a certain pH and temperature for it to react.
- Physical factors: Factors like temperature, heat, and light also play a significant role in rancidification. Heat and light are the major sources for the production of free radicals. Heat and light accelerate the process of oxidation.
How To Prevent Rancidity?
To retain the desirable qualities of food products, preventing them from becoming rancid is essential. One of the easiest ways to do this is by keeping them away from direct contact with light and air. You can store them in airtight containers for this purpose.
Adding antioxidants is also an effective way to prevent auto-oxidation in foods that contain fats and oils. Antioxidants can be either natural or synthetic. Natural antioxidants include vitamin C, vitamin E, flavonoids, and polyphenols. Sequestering agents such as EDTA also prevent or slow down oxidation and, therefore, can help effectively prevent rancidity.
Last Updated By: Ashish Tiwari
References (click to expand)
- Oxidation of food grade oils - Oils, fats and more.
- Esfarjani, F., Khoshtinat, K., Zargaraan, A., Mohammadi‐Nasrabadi, F., Salmani, Y., Saghafi, Z., … Bahmaei, M. (2019, June 6). Evaluating the rancidity and quality of discarded oils in fast food restaurants. Food Science & Nutrition. Wiley.
- Oils: Tips for Storage and Preventing Spoilage.
- LSU Scholarly Repository | Louisiana State University Research.