Table of Contents (click to expand)
Aerogels are a diverse class of solid, porous materials exhibiting an uncanny assemblage of extreme material properties and extremely light weight.
Have you ever dreamt of sleeping on fluffy white clouds or diving in a pool of solid air, only to swim in its strange sheer mushy texture. Aerogels do look a lot like your fantasy wool. However, they are very different. Being the lightest material in existence, to date, aerogels are unconvincingly sturdy and incredibly immune to a wide range of harsh conditions.
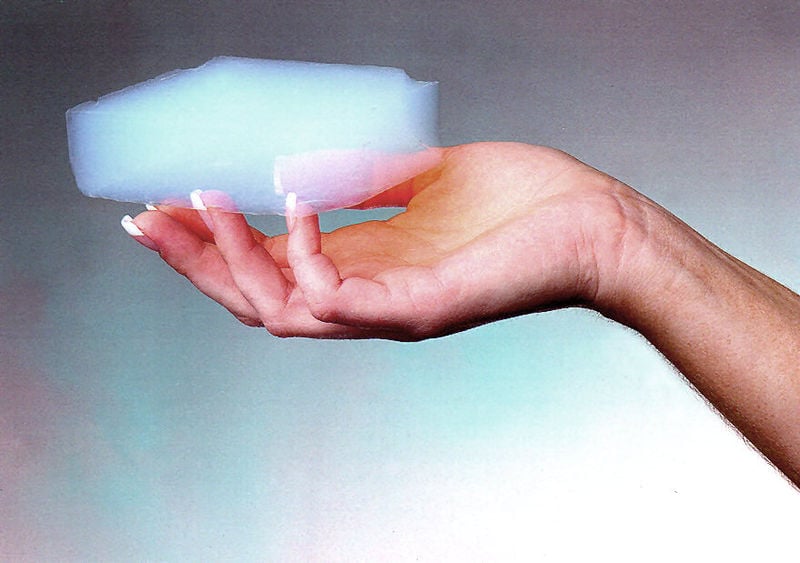
“Aerogel” cannot be deemed as a specific material or mineral, such as cotton or graphene, and have a specific chemical formula. Instead, they are a diverse class of solid porous materials exhibiting an uncanny assemblage of extreme material properties, consisting of a group of materials with a particular geometrical structure—an extremely porous solid foam with a high linkage between branched structures across the material. These linkages, although spanning a few nanometers, are incredibly strong and durable. Much to your surprise, these “mystically flamboyant” materials have been around throughout history for much longer than you may imagine. American chemistry professor Samuel Kistler, in 1931, published his first findings concerning this material after successfully inventing it, a process that included a lot of trial and error.
Recommended Video for you:
How To Make Aerogel?
Imagine preparing a bowl of a sweet gelatin dessert. The process for preparing aerogel is actually quite similar. The gelatin powder is mixed in hot water and then cooled in a refrigerator. What you get is a gel. At this point, the aerogel and your regular edible jello are no different. If you placed this wiggly gel in an oven now and removed all of its moisture, your jelly would undoubtedly turn to powder. This is because, when the moisture is being siphoned out as steam, the structural bonds between the jelly material are pulled inwards, leaving behind nothing but dust.
However, if you somehow managed to pull out all the liquid content of the gel, without damaging the solid structure and shape, what you would be left with is a low-density, extremely porous solid. This is precisely how aerogels are made.
So, how do you pull out the liquid without damaging the solid? Here’s a video demonstrating a DIY aerogel.
The Answer: Supercritical Drying
Supercritical drying is an intricate technique by which liquid can be pulled out by capillary action. All pure substances (that won’t decompose) have a critical point. This is a specific pressure and temperature at which the distinction between their liquid state and gas phase disappears. This phase of matter is called a supercritical fluid.
To create the aerogel, take a sealed container containing a liquid (mostly silica) below its critical point. This jar is equipped with a pressure gauge on top and equipment to raise the temperature. A certain amount of liquid evaporates in the container until the vapor pressure of the liquid and the pressure in vessel equalizes. Now, if you heat the container, the pressure in the container increases, due to the increase in vapor pressure with temperature. As the critical point of this liquid draws near, the pressure in the tank squeezes the vapor molecules close enough together such that the vapor becomes almost as dense as a liquid.
Simultaneously, the temperature in the container becomes high enough so that the kinetic energy of the molecules in the liquid deluges the attractive forces that hold them together as a liquid. Eventually, the critical point is reached, blurring the meniscus dividing the two phases, resulting in a single supercritical phase! In this phase, the surface tension in the fluid slowly drops to zero, forcing the capillary stress to also drop.
Aerogelification
The supercritical fluid now presents throughout the entire vessel has its pores filled with gel; the liquid in this gel can now be removed, with no surface tension to hinder the process. This is done by partially depressurizing the vessel (not below the critical pressure). The temperature of the container must also remain above the critical temperature during this step.
The objective is to get rid of enough fluid from the vessel whilst the fluid is still supercritical, which ensures that when the vessel is fully depressurized below the fluid’s critical point, there will simply be no substance left in the vessel to re-condense. Once enough fluid has been eliminated, the vessel is slowly depressurized and cooled back to ambient conditions. As this happens, the small amount of fluid left in the vessel passes back through the critical point; it reverts to a gaseous state. The remaining liquid in the gel has now been completely converted to gas, without capillary stress ever arising, and what’s left behind is the Aerogel.
Types Of Aerogel
The three basic types of aerogels are silica, carbon and metal oxides. These solids have found a wide range of applications in modern-day pieces of equipment, due to their unique structural and chemical properties.
Silica should not be confused with silicon, the substance used in microchips and semiconductors. Silica is a glassy material used for insulation. Silica aerogels are the most commonly discussed aerogels; if you hear people talking about aerogels, there’s a good chance they’re talking about silica aerogels. Silica’s molecules, being larger than the wavelength of white light, scatter blue light, thus providing a translucent blue tinge.

Quite unlike the sky-blue silica aerogels, carbon-based aerogels have a greyish black tinge with a charcoal-like texture. Their unappealing look is made up for by their high electrical conductivity properties. Millions of air gaps and pores dramatically increase the surface area of absorption of these aerogels, making them an essential candidate for fuel cells, desalination systems and supercapacitors.
Metal oxide aerogels, as the name suggests, are made from metallic oxides. They are, in fact, the inorganic cousins of the more common silica aerogel. Each type of aerogel has its own unique properties. These aerogels are highly useful as catalysts for many different chemical transformations, explosive matrices and antecedents for other materials, such as carbon nanotube catalyst. These aerogels are often quite colorful, and some are even magnetic in nature.
Applications Of Aerogel
Owing to their low thermal conductivity and extremely light weight, they are the perfect candidates for building construction and appliance insulation, storage media, automobiles and space vehicles, solar devices and solar ponds.
Thanks to their high porosity and low density, they are used in catalysis, machine sensors, fuel storage, ion exchangers, filters in exhausts, pigment carriers and templates. Being mildly translucent solids with a low refractive index, they are also used as light guides and find application in lightweight optics.
Being acoustically opaque to sounds, they are used when lining the walls of soundproof rooms and in ultrasonic distance sensors. Being lightweight and mildly elastic, they find good use as energy absorbers in hypervelocity particle traps. Having high surface area and low dielectric constants, aerogels are often used in dielectrics for ICs and capacitors because of their high dielectric strength.
As you can see, this unique class of material can do a lot more than keep the moisture out of a shoe box!