Table of Contents (click to expand)
NADP+/NADPH is a cofactor that is involved in anabolic processes, also known as biosynthetic reactions. It donates electrons (reduces) to molecules.
ATP is the star of every biology textbook, as it is the energy currency of the cell. Every metabolic reaction in the body is or leads up to a transaction of ATP, at some point. It is the cell’s superstar, and with good reason. It is comparatively small and versatile, and thus able to participate in countless processes and provide energy to any molecule that requires it. However, there is another hero, a dark horse that keeps the cells and the entire organism running. Meet NADP+.
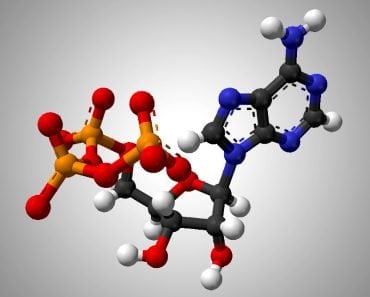
NADP+ and its reduced form, NADPH, is short for Nicotinamide Adenine Dinucleotide Phosphate (we’ll stick to NADP+, thanks). It looks very similar to NAD+ (Nicotinamide Adenine Dinucleotide), but without the phosphate. Their structure makes them uniquely useful to many enzymes as a cofactor in their reactions. A cofactor is any non-protein molecules or metal ions required by the enzyme to complete its job. NADP+ is a transient cofactor, which means that it isn’t covalently linked to the enzyme. Instead, it is more like a substrate, in that it interacts with the enzyme, helps it carry out its function and then leaves.
Recommended Video for you:
What Is NADP+/NADPH?
NADPH is an electron carrier. It accepts energized electrons released during some metabolic reactions. NADP+ and other such cofactors (NAD+ and FAD+) are capable of accepting these electrons in a stable manner without forming harmful and overly reactive radicals. They are capable of harboring 2 electrons because of the nicotinamide present in its structure. These electrons are given in the form of a hydride ion (H–), a hydrogen with one extra electron. Accepting the electrons converts NADP+ to its reduced form NADPH.
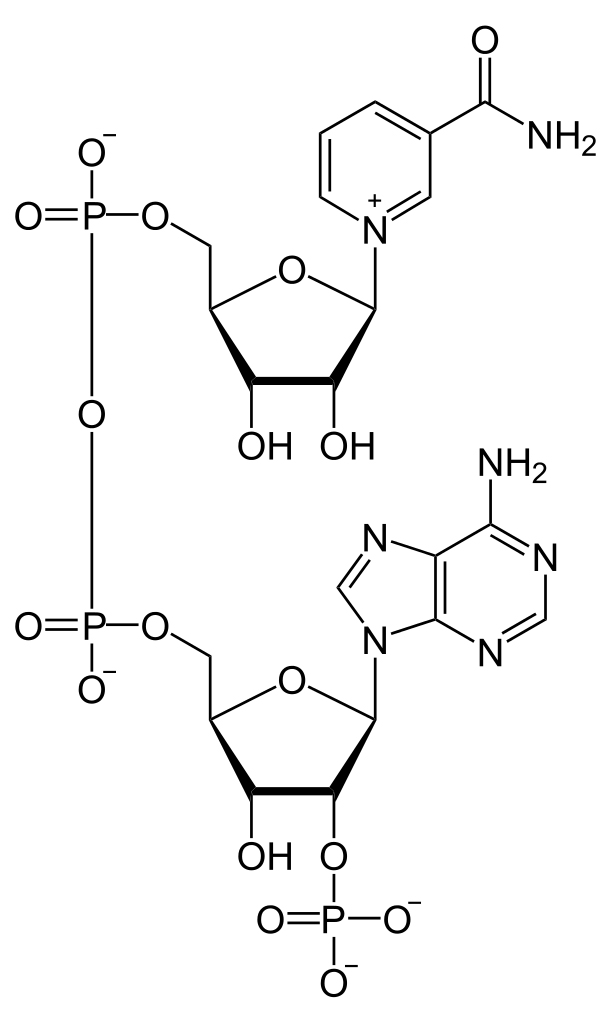
As the name suggests, they are simply electron carriers, implying that they are going to drop these electrons at a given destination. These cofactors donate their electrons to other molecules either to create energy or to build molecules. This is essentially a redox reaction.
What Is Its Role In The Cell?
All metabolic reactions are redox reactions. There is a barter of electrons between substrates, which is the reaction being catalyzed by an enzyme. One substrate gives up its electrons, becoming oxidized, while the other substrate gains those electrons, getting reduced. The mnemonic OIL RIG is useful to remember this – Oxidation Is Loss, Reduction Is Gain. In glycolysis, the pathway that breaks down glucose for energy, glucose loses electrons in a series of oxidative steps while cofactors like NAD, FAD or ADP get reduced, thus gaining those electrons.
There are two arms of metabolism – the one that makes and the one that breaks. The metabolic pathways that make new molecules or biosynthesize are called anabolic pathways. The metabolic pathways that break down molecules either to generate ATP or to break down waste are called catabolic pathways. Anabolism and Catabolism are delicately balanced within a cell. NADP+/NADPH uses its electrons to build things that are involved in anabolic or biosynthetic pathways.

In biological systems, the more reduced a molecule, the more potential it has to yield energy when it’s broken down. NADP+/NADPH’s role in the cell is to donate those electrons so that the cell can make things. One can think of it as the glue sticking things together using electrons.
NADH and FADH2, the reduced forms of NAD and FAD, rather than directly reducing molecules, are sent to donate their electrons to reduce ADP to make more ATP.
How Is NADP+/NADPH Formed?
NADPH is generated when NADP+ gets reduced. The molecule NADP is produced through certain biosynthetic pathways that differ slightly depending on the organism. The precursor for the pyridine ring (adenine) is quinolinate across all organisms, whereas the nicotinamide comes from vitamin B3. The cell first makes NAD, after which the enzyme called NAD kinase (kinases are enzymes that add phosphates to molecules) makes NADP+. This NADP+ goes on to get reduced. This reduction happens through certain metabolic pathways in the cell.
In most mammalian cells, the pentose phosphate pathway generates the bulk of NADPH. This pathway, also called the EMD pathway, is a shunt pathway that takes glucose away from generating ATP through glycolysis, and instead makes NADPH and 5-carbon sugars (ribose). This pathway primarily functions when the cell is well-stocked with ATP. With no dire need for energy, it uses this opportunity to build biomolecules, such as lipids (fats), proteins, and nucleotides—the rungs on the ladders of DNA and RNA.
The NADPH provided by the Pentose Phosphate pathway isn’t only used for biosynthesis. NADPH helps antioxidants, such as glutathione, fight oxidative stress. Oxidative stress from reactive oxygen species (ROS), generated by many metabolic reactions, can lead to malfunctioning cells, increased cellular mutations, and cell death. Overall, it could contribute to prematurely aging the organism and increase its risk of inflammation. The cell’s inbuilt reducing mechanisms defend against such stress, enhancing the ability of the cell to fight of diseases.
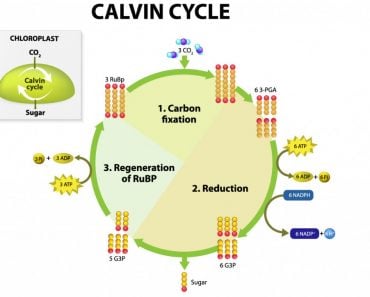
In plants, there is another contributor to the pool of NADPH – photosynthesis! Photosynthesis is the process through with plants make their food, primarily sugars. The first step in photosynthesis primes the plant cell with resources to make its food. Using water and the photons from light, plants generate ATP and NADPH in reactions called the light-dependent reactions or simply “the light reactions”. Photosystem I (PSI) supplies the electrons to the enzyme Ferrodoxin-NADP+ reductase, which reduces NADP+ to NADPH. This NADPH is then shuttled to the second half of photosynthesis, the Calvin Cycle, a.k.a the dark reactions or the light-independent reactions.
Conclusion

NADP+/NADPH is the unsung hero of the cell’s cofactors. Without its electron-carrying and -reducing powers, building essential molecules for life would be very difficult, if not impossible. NADP+/NADPH is involved in building triglycerides, cholesterol and steroid molecules, which are important in cell membranes and hormones. It also helps build nucleotides that end up as part of DNA and RNA.
The ability to construct certain molecules allows organisms to control their cellular growth and division. This ability, as well as being able to digest and break down food for nutrients, has allowed many organisms to grow to large sizes. Plants, algae, and some bacteria, without photosynthesis producing ATP and NADPH using water and light, wouldn’t have been able to flourish on early Earth as they did. This small reducing molecule serves as a protector and builder in the cell, and one of the main drivers of life!
References (click to expand)
- Ying, W. (2008, February). NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxidants & Redox Signaling. Mary Ann Liebert Inc.
- Kurnasov, O., Goral, V., Colabroy, K., Gerdes, S., Anantha, S., Osterman, A., & Begley, T. P. (2003, December). NAD Biosynthesis. Chemistry & Biology. Elsevier BV.
- Voet D., Voet J. G.,& Pratt C. W. (2015). Fundamentals of Biochemistry: Life at the Molecular Level. Wiley