Table of Contents (click to expand)
When an atom is unstable, it means that it doesn’t have the right number of protons and neutrons in its nucleus. This can happen when the atom has too many or too few of either. When this happens, the atom releases radiation in an attempt to become stable. This radiation can be harmful to people if they are exposed to it for a long time.
You probably have heard the term “radioactive” a number of times in your life, especially while interacting with students, or perhaps a friend who works at CERN (an unlikely scenario), someone who’s a fan of Madam Curie, or a person who frequently listens to the American rock band Imagine Dragons. It would be fair to say that the general perception of the word “radioactive” is mostly unsavory, unless you’re one of those people who totally believe in the fictionalized benefits of being bitten by a radioactive spider, or being exposed to powerful radiation.

Despite being consistently portrayed in the media and popular culture, many people don’t really know what radioactivity as a phenomenon is all about. In this article, we will look at the basics and try to understand what it takes for something to be radioactive!
Important note: Radioactivity as an idea is truly immense. It’s so extensive and complex that scholars decided to put it into a separate field of Physics altogether. Explaining it in its entirety in a single post is about the same as climbing Mt. Everest in an hour, i.e., impossible. So, keep in mind that the following text deals only with a very specific segment of the mammoth concept that radioactivity actually is.
First things first…
Recommended Video for you:
What Is Radioactivity?
The term might sound somewhat technical and even invoke a negative feeling in some, but it is rather straightforward to understand. It is the process through which the nucleus of an unstable atom transforms into a stable one, along with releasing energy in the form of radiation. A few other terms, such as nuclear decay or radioactive decay, also mean the same thing and are thus used interchangeably. A material is said to be radioactive if it spontaneously emits this kind of ionizing radiation.

Contrary to popular belief, the presence of radioactive materials is not only limited to high-tech chemical laboratories; rather, radioactivity is a part of the Earth and has existed since its birth. Radioactive materials are found naturally in the Earth’s crust, rocks, soil, ocean water etc.
Not only that, but radiation is also present in our buildings, walls and floors; even our own bodies play host to certain naturally-occurring radioactive elements. Suffice to say that one cannot EVER be in a “radioactivity-free environment”.
A Little Something About Elements, Atoms And Subatomic Particles
Everything around you, and everything that exists in the universe, is composed of a combination of different basic substances that scientists like to call “chemical elements”. As of now, there are 118 elements known to humans; 94 occur naturally on Earth and the remaining 24 are synthetic ones, made by humans at some point in recent history.
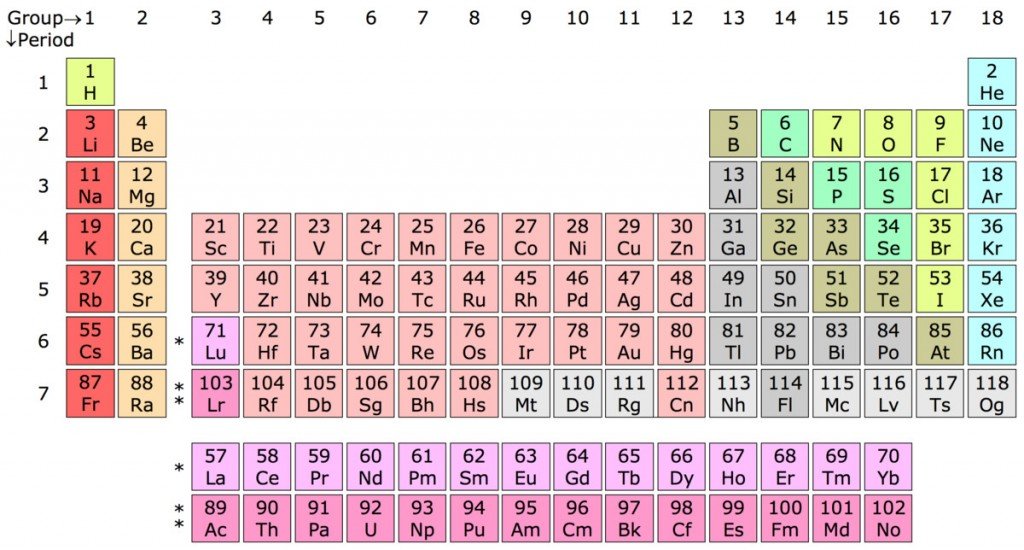
Elements are made of super-tiny particles known as atoms. You might have read about these in high school science classes. Atoms are composed of three major subatomic particles – electrons, protons and neutrons. Of these three, the latter two sit inside the nucleus of the atom.
What Makes Something Radioactive?
Stable Versus Unstable Atoms
Whether an atom is radioactive or not depends entirely on its stability. Stability, in the context of atomic nuclei, pertains to the balance of the internal forces among particles. You see, there are a given number of protons and neutrons in a nucleus which, under normal conditions, coexist without losing any energy. Such an atom is considered stable. In other words, you can say that a “stable” atom is one that is happy with its current internal energy.
However, there are certain atoms that either have too many or too few neutrons or protons in their nuclei. This results in an imbalance between the forces holding them together, which leads to an excess of internal energy. Such atoms are said to be unstable or radioactive.
In a bid to attain stability, these atoms throw off protons or neutrons to reduce their mass, which are converted into energy (think Einstein’s energy-mass equivalence) and emitted in the form of ionizing radiation (like alpha, beta and gamma radiation). Prolonged exposure to this type of radiation is harmful.

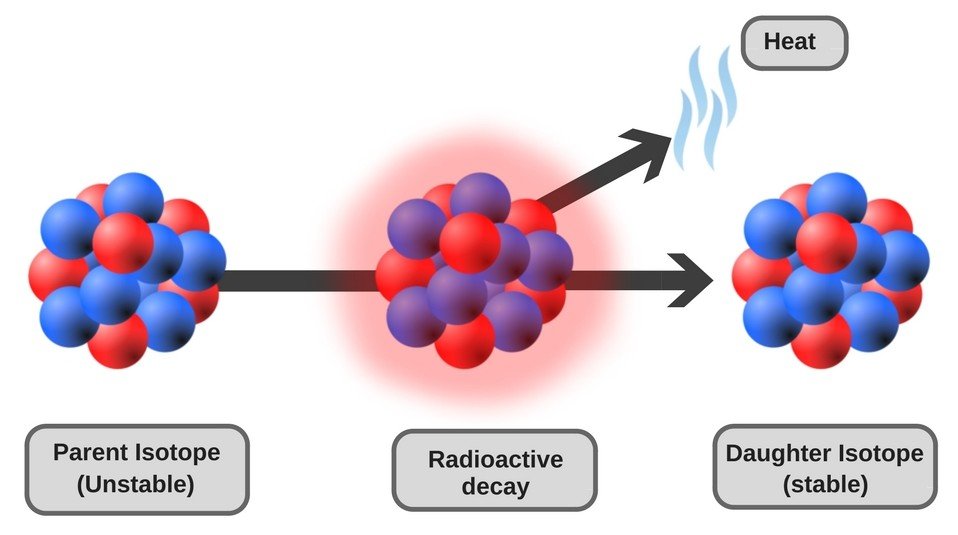
Radioactive Decay
Once a radioactive nucleus gets rid of the excess mass in the form of ionizing radiation, it transforms into a different nuclide (a stable version of the nucleus characterized by a specific number of protons and neutrons) and attains stability. The emission of ionizing radiation continues until there is no longer an imbalance between the internal forces of the nucleus. This process of decay of the nucleus to attain stability is called radioactive decay.
Ionizing radiation, in essence, is just a “part” of an atom that flies off at high speeds. The biggest pieces of these bits are alpha particles, which are akin to cannonballs. They are big and heavy (as “big” as a “part” of an atom can be) and do significant damage to any surface they hit. Next come beta particles, which are akin to bullets. They are smaller, but faster than alpha particles, and do less damage. The third category is gamma particles, which are like lasers. They don’t weigh anything at all and are the most difficult to stop. You’ll need concrete, lead, or other heavy shielding to stop them from penetrating a surface.
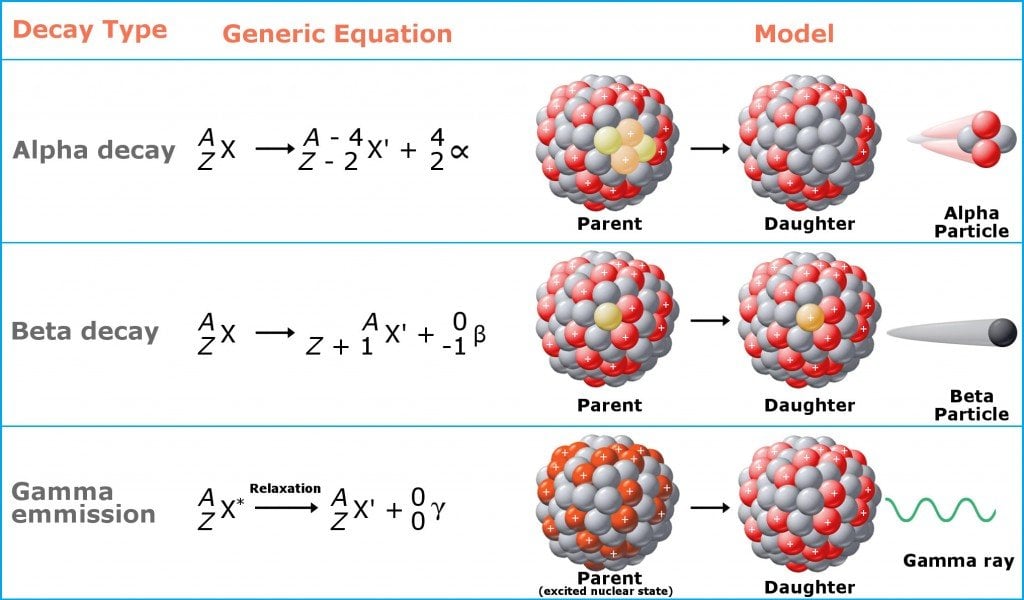
As mentioned earlier, this post is just the tip of the iceberg that radioactivity really is. For more detailed information on radioactive decay, radiation and the dangers it poses, check out the links provided in the next section.