Table of Contents (click to expand)
Water droplets seem to dance around the surface of a hot pan due to ‘The Leidenfrost Effect’. This effect results in the formation of a vapor cushion between the liquid and the surface, which keeps the droplet eerily aloft.
When you’re cooking something, how do you know if the cooking pan is hot enough to begin? One foolproof method to check this is by sprinkling some water droplets on the pan. If the droplets fizzle out immediately into water vapor, it means that the pan is hot; but not hot enough. Instead, if the water droplets float and zoom around the pan for a while before evaporating, it means that your pan is super-hot and ready for some hardcore culinary experimentation.
This simple trick has been in practice in our households for a very long time, but something about it doesn’t seem right. We know that water evaporates at around 100oC, so logically, at higher temperatures, evaporation would happen even faster, right? If that’s true, why does liquid water scamper around on surfaces that are so much hotter than its boiling point?
Recommended Video for you:
What Happens To Water On A Hot Surface?
The boiling point of water is around 100oC at sea level, but why exactly does water boil at this particular temperature? Temperature, in simple terms, is a measure of the kinetic energy of the measured particles. This means that at higher temperatures, the particles have higher kinetic energy. Consequently, they move around more vigorously, break off their bonds more easily, and gradually expand out to their vapor state. This is why water vaporizes when in contact with a body around its boiling point.
If you place a drop of liquid water on a surface whose temperature is a little lower than its boiling point, the drop will flatten out and slowly get heated. However, if the surface is right around the boiling point, we observe that the drop gets evaporated almost instantly, along with a hissing sound.
Now, what happens when the surface is way hotter than the boiling point of water? Does it vaporize immediately? Well, yes and no.
At a temperature that is significantly higher than the liquid’s boiling point, something interesting happens.
Since the surface is really hot, the moment the base of the water droplet touches the hot surface, it vaporizes. This instantaneous vaporization creates a cushion of steam between the liquid water and the heated surface. This process is called ‘Film Boiling’ and is the cause behind the ‘Dancing Water Effect’, more formally known as The Leidenfrost Effect.
The Leidenfrost Effect
This effect occurs when a liquid comes in contact with a surface that is at a temperature much higher than the liquid’s boiling point.

The Leidenfrost Effect was first described by the German theologian Johann Gottlob Leidenfrost in the 1750s in a manuscript called A Tract About Some Qualities of Common Water. He was conducting experiments by placing water droplets on a red-hot iron spoon when he noticed something fascinating; instead of boiling immediately, the drops seemed to persist on the spoon. In fact, it appeared as if the droplets were absorbing heat from the red-hot surface.
What Causes The Leidenfrost Effect?
The vapor cushion created during film boiling is what’s responsible for the Leidenfrost Effect;
Firstly, the gaseous barrier formed acts as a thermal insulator. The thermal conductivity of water vapor is almost 20 times less than that of liquid water. Hence, the vapor cushion prevents further transmission of heat from the surface to the liquid layer. This is why the water droplet remains as a liquid even at such high temperatures, but eventually, the lower layers get flash-vaporized and the drop vanishes gradually.
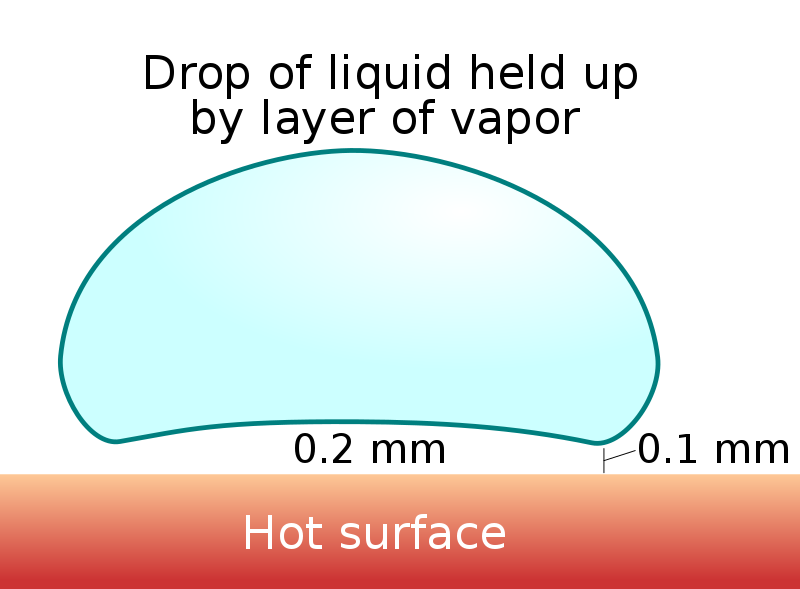
The vapor cushion formed has a thickness of around 0.2mm at the center and 0.1mm at the edges. Even if it’s really thin, this layer exerts an upward pressure that keeps the liquid drop aloft. When we see it, it looks as if the water droplet is levitating magically over a hot surface.
Moreover, the vapor layer is also sensitive to disturbances. Since the water droplet is hovering effortlessly over a cushion of gas, friction is reduced drastically. Thus, a small ridge or a slight tilt can send the droplet zooming across the surface. Now you can see why the levitating drops appear to be skittering randomly around the surface.
The temperature at which the Leidenfrost Effect occurs for a liquid is called its Leidenfrost point. This point can vary from fluid to fluid depending on the properties of the fluid and the nature of the surface. In the case of liquid water, the Leidenfrost Effect can happen anywhere from around 170oC to 220oC.
Do Other Liquids Show This Effect?
The Leidenfrost Effect can happen with any liquid that is poured over a surface whose temperature is higher than its boiling point. In fact, there are some interesting science experiments that make use of this amazing effect.
The Molten Lead Experiment
Have you ever seen people dipping their hands into molten lead and then drawing them out without getting even a single burn? The secret behind this is the Leidenfrost Effect.
The temperature of molten lead can range anywhere from 300-400oC, which is way above the boiling point of water. Before dunking their hands into the lead, people first wet their hands by dipping them in liquid water. Furthermore, when this hand is dipped into the molten lead, the water present on the hand forms a vapor layer that acts as an insulating glove over the hand, preventing it from getting burned. However, this experiment can get extremely dangerous if the hand is left in the molten lead for too long.
Liquid Nitrogen
Liquid nitrogen is another liquid that readily shows the Leidenfrost Effect. When dropped over a surface at room temperature or when spilled out into the air, liquid nitrogen appears to glide all over the surface like glass beads. This is a perfect demonstration of the Leidenfrost Effect. The boiling point of liquid nitrogen is around -196oC. The normal room temperature (20oC) is therefore way above the boiling point of nitrogen, so liquid nitrogen experiences film boiling under normal temperatures.

Applications Of The Leidenfrost Effect
Besides the cool science demonstrations, does this effect have any serious real-life applications? Yes! In fact, the Leidenfrost Effect has applications everywhere from inkjet printers to nuclear reactors.
Nuclear reactors make use of water heat exchangers to keep their temperatures in check. Here, the Leidenfrost Effect can be a villain.
If the reactors get too hot, film boiling results in the formation of a vapor layer that, in turn, hinders the heat transfer from the reactor to the water. This makes the heat exchanger less efficient and affects the proper functioning of the reactor. Therefore, in nuclear reactors, the Leidenfrost Effect must always be kept under check to prevent nuclear disasters, such as what happened in Fukushima.
Moreover, studies regarding the Leidenfrost Effect are being conducted extensively to explore more about its levitation and motion properties, which can be vital in developing self-propulsion mechanisms.
We can also make use of the Leidenfrost Effect to manipulate the movement of fluids. Scientists have found that if repeating sharp ridges are created on the hot surface, the droplets can move up the ridges just as one climbs a staircase.
Also, special mazes created using grooved surfaces and hydrophobic coatings are used to navigate water droplets across desired paths. This kind of controlled fluid motion has some interesting applications in electric current generation and fluid dynamics. Thus, the future scope of the Leidenfrost Effect is limitless.
The Leidenfrost Effect is an example of the odd nature of science and thermodynamics. Now that you understand the science behind this cool phenomenon, you have officially unraveled the power to make water droplets levitate and dance on command!
References (click to expand)
- Leidenfrost Effect - an overview | ScienceDirect Topics. ScienceDirect
- Zeuner, M., Schwark, K., Hanisch, C., & Ziese, M. (2019, October 14). Leidenfrost effect studied by video analysis. European Journal of Physics. IOP Publishing.
- Superwetting Surface for Diminishing Leidenfrost Effect Applications Problem Addressed Technology Advantages Categories For This - tlo.mit.edu
- J WALKER. BOILING AND THE LEIDENFROST EFFECT. Reed College
- G Wells. A Leidenfrost Engine - NASA/ADS. The SAO/NASA Astrophysics Data System











