Table of Contents (click to expand)
The periodic table is arranged by atomic number, which is the number of protons in an element’s nucleus. The elements are arranged in order of increasing atomic number. The periodic table is also arranged by groups and periods. The elements in a group have the same number of electrons in their outermost orbital. The elements in a period have the same number of electron shells.
The arrangement of the periodic table was formulated in order to give a very informative representation of the chemical elements. Each of these elements is specifically placed in the periodic table, keeping specific parameters in mind.
The arrangement of the periodic table has changed over time since its inception. This is because new elements have either been discovered or made by man, and later added to the periodic table.
Before we dive into the intricacies of the periodic table, let’s first take a walk down memory lane.
Recommended Video for you:
Dmitri Mendeleev – Father Of The Periodic Table
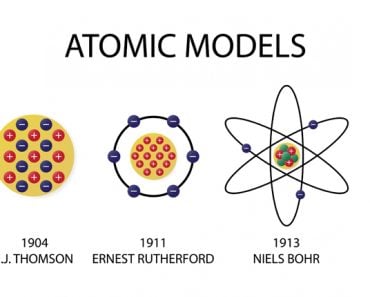
Dmitri Mendeleev, a Russian chemist and inventor, is considered the “father” of the periodic table, according to the Royal Society of Chemistry. During the 1860s, Mendeleev was a popular lecturer at St. Petersburg University in Russia. During this time, there were no authoritative modern organic textbooks that could be used as staple reading material. For this reason, Mendeleev decided to write one and tackle two primary issues: the first being to organize the disparate natures of the elements and the second being to solve the dilemma of an authoritative book.

However, this wasn’t as straightforward as one might imagine in the time of Mendeleev, as there were numerous obstacles to overcome. At that time, less than half the current elements were known, and some of those elements that were present had the wrong data. We could try understanding what Mendeleev went through by giving the analogy of a tough jigsaw puzzle with half the pieces bent out of shape.
Mendeleev ultimately succeeded in his endeavor and wrote the definitive chemistry book of his time, titled “Principles of Chemistry”, which was made into two volumes. While working on the periodic table, he ended up making the most significant contribution to organic chemistry in history. He accomplished this by writing down the properties of each element on a card in an ordered fashion of increasing atomic weight, according to the Royal Society of Chemistry. By doing this, he noticed a particular pattern occurring in the elements.
After intensely trying to work through the pattern for three days, he said that—in a dream—he saw all the elements fall into the right place. He immediately woke up and wrote it down on a piece of paper and went back to sleep. Upon waking, he found that it only needed one correction! Mendeleev arranged the elements both in terms of their atomic weights and valence. He was also smart enough to leave space for new elements that he thought would be discovered (and he was right!). He also predicted the properties of five unknown elements and what their compounds would be, even before their discovery, based on the principles of the periodic table he had arranged.
Reading The Periodic Table
Now, the periodic table can be read in any number of ways, providing a great deal of information about a particular element or group of elements.
Atomic Number
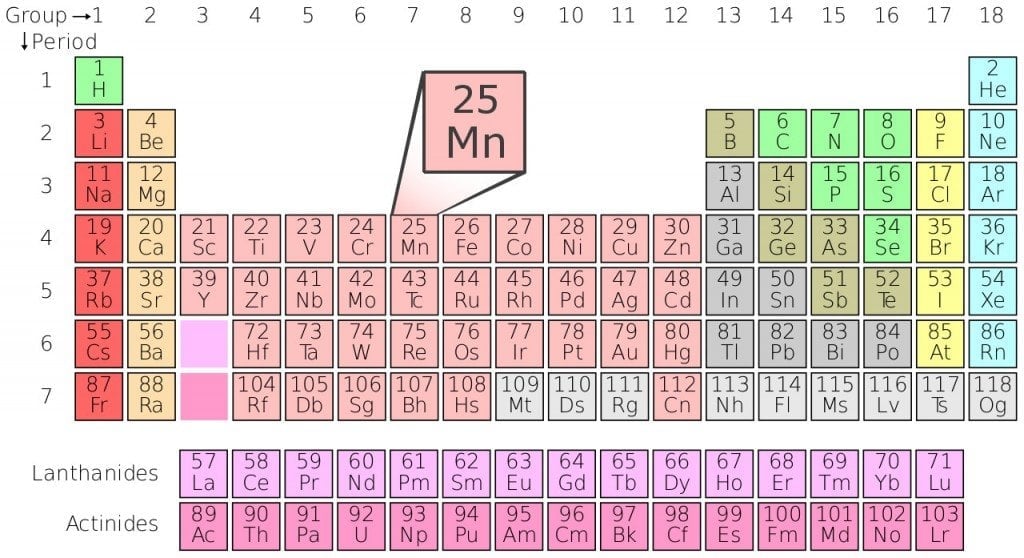
The first and most crucial aspect one can infer from the periodic table is an element’s atomic number. The number of protons in an atom is referred to as the atomic number of the element. It also gives a clear indication of the chemical behavior of the element. For example, we can say that carbon atoms have six protons, hydrogen atoms have one and oxygen has eight.
Atomic Symbol
The next crucial aspect is the Atomic Symbol. As mundane as this sounds, the atomic symbol gives us an abbreviation of every element. These symbols are accepted internationally and help us in shortening the length of the element names. The abbreviation is useful because it helps us write chemical equations faster and helps us remember the elements with greater ease.
Atomic Weight
The third crucial factor is Atomic Weight. The standard atomic weight of an element is also known as the atomic mass or atomic mass unit. Individual atoms always have an atomic number as integers, but an oddity occurs in the atomic mass given in the periodic table, as these come in the form of a decimal. This is because the atomic mass represented in the periodic table is the average atomic mass of all the isotopes. The number of neutrons present in an element can be found by subtracting the atomic number from the atomic mass (for naturally occurring elements).
Other Interferences
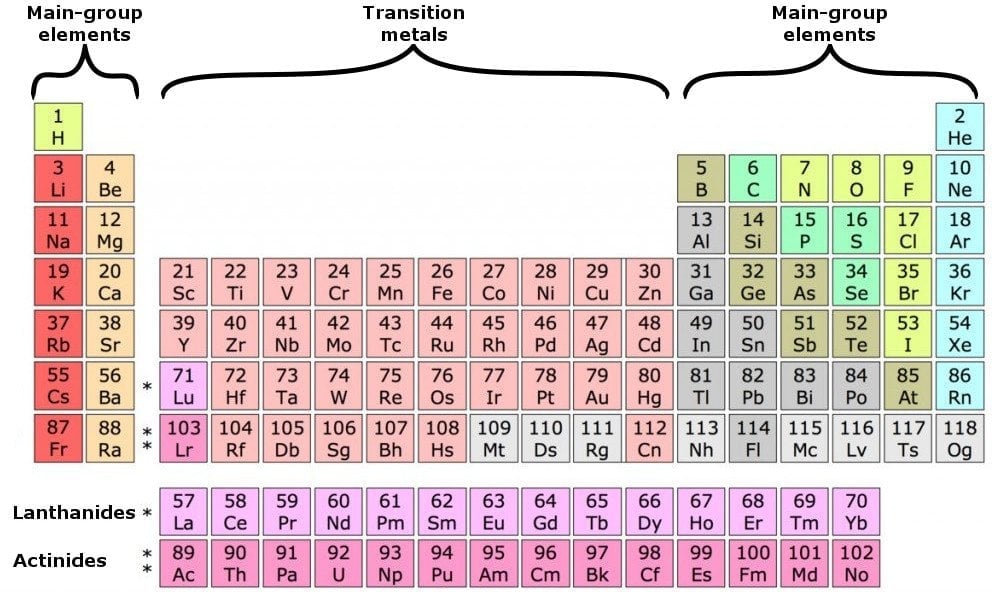
Periods And Groups
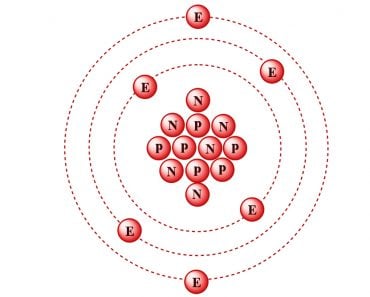
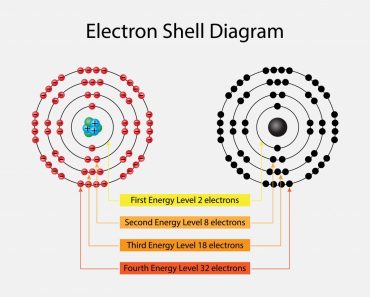
The periodic table is a graphical layout that not only gives information about individual elements, but also elements that have similar properties. The seven rows in the periodic table are known as the Periods. Each element in a particular row has the same number of electron shells surrounding the atomic nucleus.
The elements hydrogen and helium have a single orbital shell; elements in the second row of orbitals have two orbital shells and so on. The next inference we can make is from the 18 columns, known as groups. All the elements in a group have the same number of electrons orbiting the nucleus in the outermost shell. The exceptions to this rule include hydrogen, helium and the “transitional elements,” which occupy groups 3 through 12. Elements in a group share important chemical characteristics. Group 18, for example, includes the “inert” or “noble” gases. Group 17 consists of five halogens.
Graphic Indicators
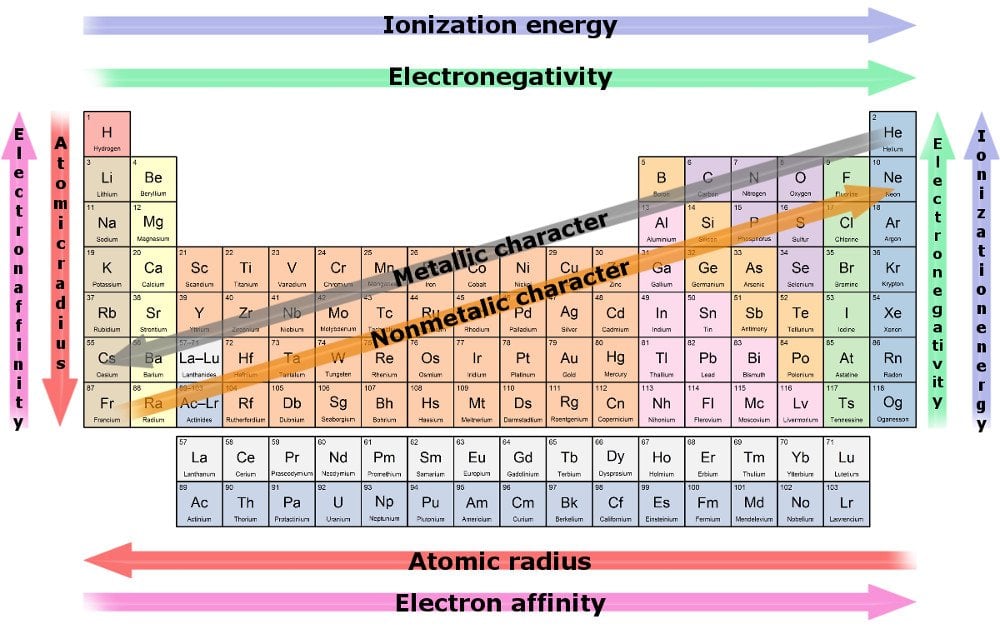
Some periodic tables also have something known as graphic indicators, which are nothing but color-coded periodic tables. These colors represent the state of the element at zero degrees Celsius. The border of the element may hint at what kind of element it is. If it has a solid border, it’s a naturally occurring element, whereas if it has a dotted border, it is radioactive, and if it contains dashes, it is a human-made element. A single thick line may appear in the middle of the periodic element in which the left-hand side is for metallic elements, and the right-hand side is for non-metallic elements.
Lanthanides And Actinides
At the bottom of the periodic table is two additional rows of elements containing a total of fourteen elements. The top row elements are called Lanthanides or rare earth elements. The bottom row of elements are called Actinides. These begin at element number 90 and end at 103. However, there are elements beyond 103, as well as a continuous effort to push for higher order elements. In conclusion, we can say that what started as the endeavor of a single man has today led to a beautiful, illustrative and highly explanatory road maps for all the known elements—and a pattern of prediction towards those elements that still remain beyond our grasp!