Table of Contents (click to expand)
Vinegar helps to dissolve iron oxide (red-brown marks) that form on stainless steel, while aluminum reduces iron oxide to iron and aluminum oxide.
Every now and then, we come across a life hack that baffles us all. I was recently floored by a trick for how my mom used to clean her stainless steel pan. She had prepared some burnt garlic chicken for dinner earlier that week. The chicken turned out great, but the pan did not fare so well. The pan had developed burn marks all over its surface. Without giving it a second thought, my mom used some regular vinegar and aluminum foil to clean the pan. With just a few scrubs, the pan looked as good as new.
How did vinegar and aluminum foil help clean off those nasty burn marks? Is my mother some kind of alchemist? Or just smarter than me? The latter is definitely true, but let’s dig in about the former.
Recommended Video for you:
What Happens When You Cook Food With Stainless Steel Utensils?
First of all, let’s take a quick lesson on stainless steel.
Stainless steel is not a naturally occurring substance. In fact, stainless steel is an alloy of steel mixed with elements like chromium, nickel, aluminum, and carbon. An alloy is a combination of two different metals or metals and non-metals. Alloys have properties that are different from their constituent elements. Thus, by alloying, the desired properties of one constituent can be retained, while its weaknesses can be overcome by adding another metal or non-metal.
Iron and carbon are the main components of stainless steel. Their combined properties make stainless steel corrosion-resistant and highly ductile. Also, it makes them an ideal choice for cooking utensils.

Now, what actually happens when you cook food in a stainless steel vessel?
When you’re cooking food in a stainless steel vessel, you are unknowingly setting up the reaction of stainless steel and oxygen in the presence of heat. The high temperature facilitates the oxidation of the iron present in stainless steel. Iron combines with the available oxygen to form iron oxides.

The end product of the reaction is a rusty-looking material (iron oxide) on the surface of the vessel. These iron oxides are the substances that you and I (but not my mom), have difficulty getting rid of.
Well, if the iron oxides are so difficult to eliminate, how do vinegar and aluminum make it disappear so easily?
How Do Vinegar And Aluminum Foil Help Clean Stainless Steel?
Vinegar and aluminum foil both play an equally important role in cleaning stainless steel. Vinegar helps to dissolve iron oxide, while the aluminum foil ensures that the dissolution continues until the very last molecules of iron oxide have been dissolved. Thus, the cleaning process occurs in two steps or two chemical reactions. The first one is between iron oxide and vinegar, while the second is between iron oxide and aluminum.
Role Of Vinegar
Vinegar is a solution of acetic acid combined with various flavoring ingredients. Commonly used varieties of vinegar, such as apple cider vinegar and white vinegar, contain 6-8% acetic acid. Acetic acid itself is a weak acid, but is strong enough to dissolve some metal oxides.
The vinegar reacts with iron oxide to form iron acetate. The chemical equation for the reaction of Iron Oxide and Vinegar is:
Fe2O3 + 6CH3COOH → 2(CH3COO)3Fe + 3H2O
(Fe = Iron, O = Oxygen, H = Hydrogen, and C = Carbon. The chemical formula for iron oxide is Fe2O3 and for vinegar it is CH3COOH)
As you can see, each molecule of iron oxide requires six molecules of vinegar to dissolve. As a result, the solution gets saturated rather quickly, while the amount of iron oxide dissolved is minimal. Once saturated, the first reaction is said to be in an equilibrium state. The iron oxides no longer continue to dissolve. This is where aluminum, ‘The Disrupter of Equilibriums’, comes into play.
Role Of Aluminum
Aluminum helps to continue the dissolution of iron oxide. Oxygen has a greater affinity for aluminum than iron, so when iron oxide comes in contact with aluminum, it loses oxygen to aluminum. The end product of the reaction is iron and aluminum oxide. The chemical equation for that reaction is:
Fe2O3 + 2Al → 2Fe + Al2O3
(Here, Al = Aluminum. Al2O3 is aluminum oxide)
Aluminum is more reactive than iron and thus oxygen, naturally, has a greater affinity for aluminum.
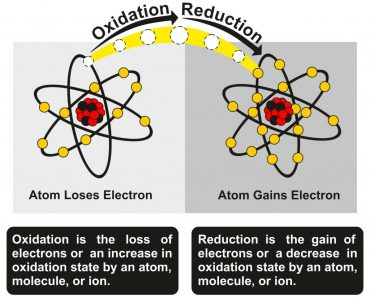
Here, iron is said to have undergone reduction. A reduction reaction is a reaction in which an element loses oxygen molecules. Aluminum gains these oxygen molecules and thus undergoes oxidation. A reaction in which oxidation and reduction occur simultaneously is called a Redox reaction. Thus, the reaction between iron oxide and aluminum is a redox reaction.
As aluminum reduces iron oxide to iron, the concentration of iron oxide in the solution decreases. This disturbs the equilibrium of the first reaction. Now, to reach equilibrium again, more iron oxide molecules are dissolved in vinegar. This cycle goes on until there are no more iron oxide molecules left.
Conclusion
That is how some regular vinegar and food-wrapping aluminum foil can help clean stainless steel. The cleaning process is a combination of displacement and a redox reaction. Iron displaces hydrogen in vinegar to form iron acetate, while the reaction between iron oxide and aluminum is a redox reaction. The two reactions together help dissolve and thus wash off all the iron oxide marks from stainless steel. Vinegar alone can also be used to clean superficial rust marks, but for big jobs, the combination of the two is king!