Table of Contents (click to expand)
The atmospheric CO2 concentration increased from 369.55 ppm in 2000 to 389.90 ppm in 2010 (Source: ftp://aftp.cmdl.noaa.gov). The change in concentration implies addition of 154.5 Gt CO2 to the atmosphere from 2000-2010. The atmospheric lifetime of CO2 molecules is 50-200 years.
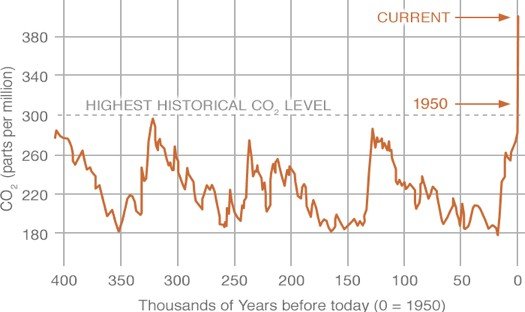
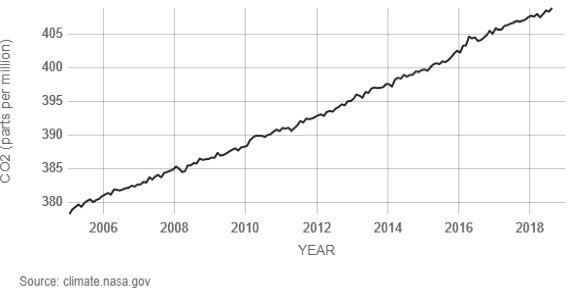
Carbon dioxide (CO2), a trace gas, is the most important greenhouse gas in the atmosphere. Similar to other gases, the concentration of CO2 is measured in parts per million, i.e., number of moles of CO2 per million moles of air. In the past 400,000 years, the concentration of CO2 varied between 200 ppm to 280 ppm. Since the mid-19th century, the concentration of CO2 has been rising and reached 410 ppm in June 2018. In the late 1950s, the annual rate of increase of CO2 concentration was about 0.73 ppm per year, and from 2005-2014 the increase was about 2.11 ppm per year (Source).
Recommended Video for you:
CO2 concentration and emission:
The concentration of CO2 is rising because the rate of CO2 emission in the atmosphere is greater than the rate of its absorption creating an imbalance in the carbon cycle. The carbon dioxide emission in the atmosphere comes from both natural and human activities.
The primary source of increased CO2 emissions is the increase in the burning of fossil fuels to meet the energy demand. The emission of carbon dioxide is measured by weight in tons of CO2. The amount of CO2 by weight can be calculated using the molecular weight of carbon dioxide and molecular weight and the total weight of air in the atmosphere.
The molecular weight of CO2 is 44 g/mole, and that of air is 28.97 g/mole. The total mean mass of the atmosphere is approximately 5×1015 tonnes. At the concentration of 400 ppm, the amount of CO2 in the atmosphere by weight is 3,000 Gt of CO2 (400 x 10-6 x 44/28.97 x 5 x 1015). The CO2 concentration increased from 369.55 ppm in 2000 to 389.90 ppm in 2010 (Source: ftp://aftp.cmdl.noaa.gov).
The change in concentration implies addition of 154.5 Gt CO2 to the atmosphere from 2000-2010. As per the data on historical emissions, the total emission in this period was 344 Gt CO2 (Source: https://www.climatewatchdata.org) indicating that the carbon sinks absorbed around 45% of the total emissions and the rest remained in the atmosphere. The atmospheric lifetime of CO2 molecules is 50-200 years. Hence, the CO2 emitted at a given time gets accumulated in the atmosphere for a long period.

Direct impacts of CO2 emissions:
Carbon sinks such as forests and oceans absorb the almost equal amount of the total CO2 emitted, and the rest remains in the atmosphere. There are two major effects of the carbon dioxide emissions – firstly, an increase in the concentration of CO2 in the atmosphere and secondly, an increase in the absorption of CO2 by oceans and plants. The increased concentration of CO2 in the atmosphere results in temperature rise. The increase absorption of CO2 by oceans causes ocean acidification. The increase absorption of CO2 by plants results in carbon fertilization. I have discussed the direct impacts of these three effects of CO2 emissions below
- Global Warming: The increase in the temperature is the major cause for all other changes in earth’s climate. The rise in temperatures is causing warming of oceans, melting of ice mass and increase in evaporation. These effects are having several physical impacts such as sea level rise and increased variability in weather patterns and extreme weather events. The adverse impact of these physical changes due to global warming is now becoming visible on the biological system and human systems. The magnitude of the impacts will be varying in both space and time.
- Ocean Acidification: The oceans absorb almost a quarter of CO2 emitted in the atmosphere. The excess CO2 reacts with the seawater to form carbonic acid. The acidification causes suppression of carbonate ion concentration that is essential for animals in the sea such as corals and shellfish to build bones and shells.
- Carbon Fertilization: The carbon dioxide emissions absorbed by plants increases photosynthesis thereby increasing plant growth depending on plant type and other inputs for plant growth. There is also a positive impact on water use efficiency. However, the effect of CO2 on plant growth is such that the photosynthetic rates increase with increasing levels of CO2 but then saturates. On the other hand, temperature affects photosynthesis and other growth and development phases of plants.
Conclusion

CO2 is a trace gas in the atmosphere but plays a critical role in maintaining the energy balance of our planet. The increase in sources of atmospheric CO2 due to the burning of fossil fuels and reduction in sinks due to changes in land use patterns such as deforestation for cultivation is causing a net increase in CO2 in the atmosphere. The increase in CO2 emissions causes global warming, ocean acidification, and carbon fertilization. Global warming and ocean acidification have a significant adverse impact on natural and human systems. Although carbon fertilization of plants increase plant growth and improves water use efficiency, these positive effects are expected to diminish gradually as the CO2 concentration increases in future.












